当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tuning the interlayer cations of birnessite-type MnO2 to enhance its oxidation ability for gaseous benzene with water resistance†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-09-19 00:00:00 , DOI: 10.1039/c8cy01147h Yang Liu 1, 2, 3, 4, 5 , Wenjing Zong 1, 2, 3, 4, 5 , Hao Zhou 1, 2, 3, 4, 5 , Dingsong Wang 1, 2, 3, 4, 5 , Ranran Cao 5, 6, 7, 8 , Jingjing Zhan 1, 2, 3, 4, 5 , Lifen Liu 1, 2, 3, 4, 5 , Ben W.-L. Jang 9, 10, 11, 12
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-09-19 00:00:00 , DOI: 10.1039/c8cy01147h Yang Liu 1, 2, 3, 4, 5 , Wenjing Zong 1, 2, 3, 4, 5 , Hao Zhou 1, 2, 3, 4, 5 , Dingsong Wang 1, 2, 3, 4, 5 , Ranran Cao 5, 6, 7, 8 , Jingjing Zhan 1, 2, 3, 4, 5 , Lifen Liu 1, 2, 3, 4, 5 , Ben W.-L. Jang 9, 10, 11, 12
Affiliation
|
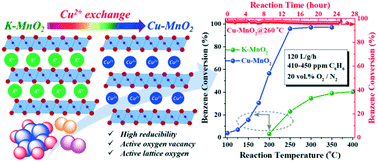
Benzene is a commonly-found air pollutant, which poses a great threat to human health and environmental development around the world. A layered birnessite-type manganese dioxide (MnO2) with a nanosheet morphology was synthesized by a simple and facile solution reaction in this study, which was further modified by Ce3+ and Cu2+ exchanges. For the pristine MnO2, benzene was hardly decomposed below 200 °C and only ∼40% conversion was achieved at a high temperature of 400 °C. However, Ce3+ and Cu2+ modifications significantly improved the activity of MnO2 for benzene decomposition. The Ce–MnO2 and Cu–MnO2 samples started to decompose benzene around 100 °C and nearly all benzene could be removed around 250 °C for ∼410 ppm benzene in dry gas under 120 L g−1 h−1 space velocity. Moreover, the Cu–MnO2 catalyst possessed the highest reaction rate (normalized by the surface area) among all the samples, and exhibited high resistance to high-temperature deactivation as well as good stability during continuous long-term testing. The origin of the tremendous effect of interlayer cations on the catalytic activity was studied via XRD, Raman, FT-IR, SEM, (HR)TEM, EDS mapping, BET, XPS and temperature-programmed techniques, which showed that the best performance of the Cu–MnO2 catalyst is attributed to it having the highest reducibility as well as highest lattice oxygen reactivity resulting from the larger number of active oxygen vacancies. Finally, the results of the humid stream reaction demonstrate that using Cu2+ exchange could significantly improve the water-resistant properties of MnO2 catalysts.
中文翻译:

调整水钠锰矿型MnO 2的层间阳离子以增强其对具有抗水性的气态苯的氧化能力†
苯是一种常见的空气污染物,对全球人类健康和环境发展构成了巨大威胁。通过简单,简便的溶液反应合成了具有纳米片形态的层状水钠锰矿型二氧化锰(MnO 2),并通过Ce 3+和Cu 2+交换对其进行了进一步改性。对于原始的MnO 2,苯在200°C以下几乎不分解,在400°C的高温下仅实现约40%的转化率。然而,Ce 3+和Cu 2+修饰显着提高了MnO 2的苯分解活性。Ce–MnO 2和Cu–MnO 2样品在100°C附近开始分解苯,在120 L g -1 h -1空速下,在干燥气体中约有410 ppm的苯,在250°C附近几乎可以除去所有苯。而且,Cu-MnO 2催化剂在所有样品中具有最高的反应速率(按表面积标准化),并且在连续长期测试中表现出较高的抗高温失活性和良好的稳定性。通过XRD,Raman,FT-IR,SEM,(HR)TEM,EDS作图,BET,XPS和温度编程技术研究了层间阳离子对催化活性的巨大影响的起源。 Cu–MnO 2催化剂归因于其具有最高的还原性以及由于大量的活性氧空位而导致的最高的晶格氧反应性。最后,湿流反应的结果表明,使用Cu 2+交换可以显着提高MnO 2催化剂的耐水性。
更新日期:2018-09-19
中文翻译:

调整水钠锰矿型MnO 2的层间阳离子以增强其对具有抗水性的气态苯的氧化能力†
苯是一种常见的空气污染物,对全球人类健康和环境发展构成了巨大威胁。通过简单,简便的溶液反应合成了具有纳米片形态的层状水钠锰矿型二氧化锰(MnO 2),并通过Ce 3+和Cu 2+交换对其进行了进一步改性。对于原始的MnO 2,苯在200°C以下几乎不分解,在400°C的高温下仅实现约40%的转化率。然而,Ce 3+和Cu 2+修饰显着提高了MnO 2的苯分解活性。Ce–MnO 2和Cu–MnO 2样品在100°C附近开始分解苯,在120 L g -1 h -1空速下,在干燥气体中约有410 ppm的苯,在250°C附近几乎可以除去所有苯。而且,Cu-MnO 2催化剂在所有样品中具有最高的反应速率(按表面积标准化),并且在连续长期测试中表现出较高的抗高温失活性和良好的稳定性。通过XRD,Raman,FT-IR,SEM,(HR)TEM,EDS作图,BET,XPS和温度编程技术研究了层间阳离子对催化活性的巨大影响的起源。 Cu–MnO 2催化剂归因于其具有最高的还原性以及由于大量的活性氧空位而导致的最高的晶格氧反应性。最后,湿流反应的结果表明,使用Cu 2+交换可以显着提高MnO 2催化剂的耐水性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号