当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of a ZrO2 support on Cu/Fe2O3–CeO2/ZrO2 catalysts for NO removal by CO using a rotary reactor
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-09-18 00:00:00 , DOI: 10.1039/c8cy01546e Xingyu Zhang 1, 2, 3, 4, 5 , Xingxing Cheng 1, 2, 3, 4, 5 , Chunyuan Ma 1, 2, 3, 4, 5 , Xiuping Wang 1, 2, 3, 4, 5 , Zhiqiang Wang 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-09-18 00:00:00 , DOI: 10.1039/c8cy01546e Xingyu Zhang 1, 2, 3, 4, 5 , Xingxing Cheng 1, 2, 3, 4, 5 , Chunyuan Ma 1, 2, 3, 4, 5 , Xiuping Wang 1, 2, 3, 4, 5 , Zhiqiang Wang 1, 2, 3, 4, 5
Affiliation
|
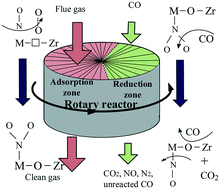
The effect of a ZrO2 support on the activity and adsorption behavior of Cu/Fe2O3–CeO2/ZrO2 catalysts was investigated by BET, XRD, SEM, Raman, H2-TPR and XPS. The NO removal performance of these catalysts in a rotary reactor at 150 °C was correlated with their catalytic activity for NO + CO, and the surface processes occurring upon NO + O2 interaction with the catalysts were studied by in situ DRIFTS. The results showed that the doping of suitable amounts of ceria into the zirconia lattice would lead to contraction of its lattice and an increase in bulk defects, which could promote the oxygen mobility and enhance the interaction between Cu/Fe2O3–CeO2/ZrO2 and ZrO2. The ZrO2 support could increase the concentration of oxygen vacancies, which could promote the dissociation of oxygen molecules and increase the activity for NO oxidation. The surface oxygen contributed to the conversion of NO2 into stable nitrates and the low concentration of surface oxygen promoted the formation of labile nitrites. The remarkably high NO removal efficiency was attributed to the significantly enhanced adsorption of unstable adsorbed NOx species (i.e., free nitrate ions and nitrosyl) and diminished stable adsorbed NOx species (i.e., bidentate nitrate and bridged nitrate) via Cu/Fe2O3–CeO2/ZrO2 support interactions.
中文翻译:

ZrO 2载体对Cu / Fe 2 O 3 -CeO 2 / ZrO 2催化剂上使用旋转反应器的CO脱除NO的影响
通过BET,XRD,SEM,Raman,H 2 -TPR和XPS研究了ZrO 2载体对Cu / Fe 2 O 3 -CeO 2 / ZrO 2催化剂的活性和吸附行为的影响。这些催化剂在旋转反应器中在150°C下的NO去除性能与其对NO + CO的催化活性相关,并且通过原位研究了在NO + O 2与催化剂相互作用时发生的表面过程。草稿。结果表明,在氧化锆晶格中掺杂适量的二氧化铈会导致其晶格的收缩和块体缺陷的增加,从而促进氧的迁移并增强Cu / Fe 2 O 3 -CeO 2 /之间的相互作用。ZrO 2和ZrO 2。ZrO 2载体可以增加氧空位的浓度,可以促进氧分子的离解并增加NO氧化的活性。表面氧有助于NO 2的转化进入稳定的硝酸盐和低浓度的表面氧促进了不稳定的亚硝酸盐的形成。的显着高的NO除去效率归因于显著增强不稳定吸附NO的吸附X物种(即,自由硝酸根离子和亚硝酰基)和减少的稳定吸附NO X物种(即,双齿硝酸盐和桥连的硝酸盐)通过铜/铁2 ö 3 –CeO 2 / ZrO 2支持相互作用。
更新日期:2018-09-18
中文翻译:

ZrO 2载体对Cu / Fe 2 O 3 -CeO 2 / ZrO 2催化剂上使用旋转反应器的CO脱除NO的影响
通过BET,XRD,SEM,Raman,H 2 -TPR和XPS研究了ZrO 2载体对Cu / Fe 2 O 3 -CeO 2 / ZrO 2催化剂的活性和吸附行为的影响。这些催化剂在旋转反应器中在150°C下的NO去除性能与其对NO + CO的催化活性相关,并且通过原位研究了在NO + O 2与催化剂相互作用时发生的表面过程。草稿。结果表明,在氧化锆晶格中掺杂适量的二氧化铈会导致其晶格的收缩和块体缺陷的增加,从而促进氧的迁移并增强Cu / Fe 2 O 3 -CeO 2 /之间的相互作用。ZrO 2和ZrO 2。ZrO 2载体可以增加氧空位的浓度,可以促进氧分子的离解并增加NO氧化的活性。表面氧有助于NO 2的转化进入稳定的硝酸盐和低浓度的表面氧促进了不稳定的亚硝酸盐的形成。的显着高的NO除去效率归因于显著增强不稳定吸附NO的吸附X物种(即,自由硝酸根离子和亚硝酰基)和减少的稳定吸附NO X物种(即,双齿硝酸盐和桥连的硝酸盐)通过铜/铁2 ö 3 –CeO 2 / ZrO 2支持相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号