当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-Terminal Domain Truncation and Domain Insertion-Based Engineering of a Novel Thermostable Type I Pullulanase from Geobacillus thermocatenulatus
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2018-09-17 00:00:00 , DOI: 10.1021/acs.jafc.8b03331 Lingmeng Li 1 , Fengying Dong 1 , Lin Lin 2, 3 , Dannong He 3 , Wei Wei 1 , Dongzhi Wei 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2018-09-17 00:00:00 , DOI: 10.1021/acs.jafc.8b03331 Lingmeng Li 1 , Fengying Dong 1 , Lin Lin 2, 3 , Dannong He 3 , Wei Wei 1 , Dongzhi Wei 1
Affiliation
|
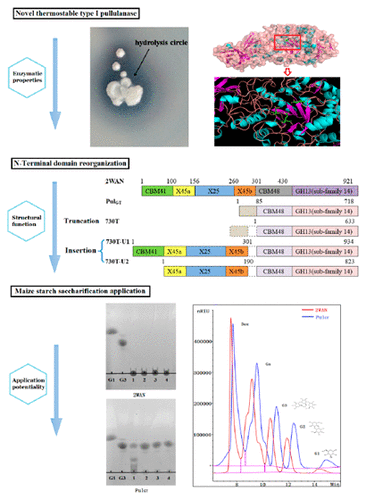
A novel thermostable type I pullulanase gene (pulGT) from Geobacillus thermocatenulatus DSMZ730 was cloned. It has an open reading frame of 2154 bp encoding 718 amino acids. G. thermocatenulatus pullulanase (PulGT) was found to be optimally active at pH 6.5 and 70 °C. It exhibited stable activity in the pH range of 5.5–7.0. PulGT lacked three domains (CBM41 domain, X25 domain, and X45 domain) compared with the pullulanase from Bacillus acidopullulyticus (2WAN). Different N-terminally domain truncated (730T) or spliced (730T-U1 and 730T-U2) mutants were constructed. Truncating the N-terminal 85 amino acids decreased the Km value and did not change its optimum pH, an advantageous biochemical property in some applications. Compared with 2WAN, PulGT can be used directly for maize starch saccharification without adjusting the pH, which reduces cost and improves efficiency.
中文翻译:

N端域截断和域插入基于工程的新型热稳定I型支链淀粉酶从热芽孢杆菌。
克隆了一个新的热稳定的I型支链淀粉酶基因(pul GT),它来自热芽孢杆菌DSMZ730。它具有2154 bp的开放阅读框,编码718个氨基酸。已发现热catenulatus支链淀粉酶(Pul GT)在pH 6.5和70°C时具有最佳活性。在5.5-7.0的pH范围内表现出稳定的活性。与来自酸解芽孢杆菌(2WAN)的支链淀粉酶相比,Pul GT缺少三个域(CBM41域,X25域和X45域)。构建了不同的N末端结构域截短的(730T)或剪接的(730T-U1和730T-U2)突变体。截断N端85个氨基酸可降低K m值,并且没有改变其最佳pH值,在某些应用中具有有利的生化特性。与2WAN相比,Pul GT无需调节pH即可直接用于玉米淀粉糖化,从而降低了成本并提高了效率。
更新日期:2018-09-17
中文翻译:

N端域截断和域插入基于工程的新型热稳定I型支链淀粉酶从热芽孢杆菌。
克隆了一个新的热稳定的I型支链淀粉酶基因(pul GT),它来自热芽孢杆菌DSMZ730。它具有2154 bp的开放阅读框,编码718个氨基酸。已发现热catenulatus支链淀粉酶(Pul GT)在pH 6.5和70°C时具有最佳活性。在5.5-7.0的pH范围内表现出稳定的活性。与来自酸解芽孢杆菌(2WAN)的支链淀粉酶相比,Pul GT缺少三个域(CBM41域,X25域和X45域)。构建了不同的N末端结构域截短的(730T)或剪接的(730T-U1和730T-U2)突变体。截断N端85个氨基酸可降低K m值,并且没有改变其最佳pH值,在某些应用中具有有利的生化特性。与2WAN相比,Pul GT无需调节pH即可直接用于玉米淀粉糖化,从而降低了成本并提高了效率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号