Journal of Colloid and Interface Science ( IF 9.9 ) Pub Date : 2018-09-17 , DOI: 10.1016/j.jcis.2018.09.053 Emily Summerton , Martin J. Hollamby , Cécile S. Le Duff , Emma S. Thompson , Tim Snow , Andrew J. Smith , Christopher Jones , Jeanluc Bettiol , Serafim Bakalis , Melanie M. Britton
|
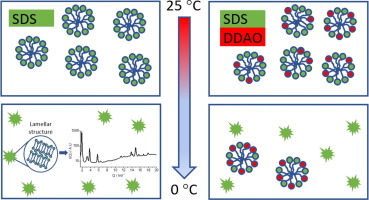
Surfactant crystallisation is important in many applications in the food, consumer product and medical sectors. However, these processes are not well understood. In particular, surfactant crystallisation can be detrimental to the stability of detergent formulations, such as dish liquid products, resulting in a turbid solution that fails appearance criteria. With the rising global demand for detergent products, understanding the factors that influence formulation stability is of increasing importance. To enable industry to build more robust formulations, it is important to understand the underlying chemistry of the crystallisation process. Here, a model system containing anionic (sodium dodecyl sulfate, SDS) and amphoteric (N,N-dimethyldodecylamine N-oxide, DDAO) surfactants, at concentrations typical of dish liquid products, is studied. Variable temperature 1H nuclear magnetic resonance (NMR) spectroscopy and small-angle X-ray scattering (SAXS) is used to probe the compositional and structural properties of this system, as a function of pH. On cooling, at pH 9, a mixture of hydrated crystals, predominately composed of SDS, and micelles containing both surfactants, have been observed prior to complete freezing. At pH 2, both surfactants appear to undergo a simultaneous phase transition, resulting in the removal of micelles and the formation of hydrated crystals of mixed composition.
中文翻译:

低温下混合十二烷基硫酸钠和N,N-二甲基十二烷基胺N-氧化物水体系的核磁共振和小角X射线散射研究
表面活性剂的结晶在食品,消费品和医疗领域的许多应用中很重要。但是,这些过程还没有被很好地理解。特别地,表面活性剂的结晶可能不利于洗涤剂制剂例如餐具液体产品的稳定性,导致混浊的溶液不符合外观标准。随着全球对洗涤剂产品需求的增长,了解影响制剂稳定性的因素变得越来越重要。为了使行业能够构建更强大的配方,重要的是了解结晶过程的基本化学原理。在此,研究了一种模型系统,该模型系统以餐具液体产品的典型浓度包含阴离子表面活性剂(十二烷基硫酸钠,SDS)和两性表面活性剂(N,N-二甲基十二烷基胺N-氧化物,DDAO)。1 H核磁共振(NMR)光谱和小角X射线散射(SAXS)用于探测该系统的组成和结构性质(随pH的变化)。在完全冻结之前,观察到在pH为9的冷却条件下,水合晶体(主要由SDS组成)和含有两种表面活性剂的胶束的混合物。在pH 2时,两种表面活性剂似乎同时发生相变,从而导致胶束的去除和混合成分的水合晶体的形成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号