当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic and Regiodivergent Mannich Reaction of Ketones with Benzoxazinones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-10-08 , DOI: 10.1002/adsc.201800870 Mayavan Viji 1 , Jaeuk Sim 1 , Siyuan Li 1 , Heesoon Lee 1 , Kyungsoo Oh 2 , Jae-Kyung Jung 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-10-08 , DOI: 10.1002/adsc.201800870 Mayavan Viji 1 , Jaeuk Sim 1 , Siyuan Li 1 , Heesoon Lee 1 , Kyungsoo Oh 2 , Jae-Kyung Jung 1
Affiliation
|
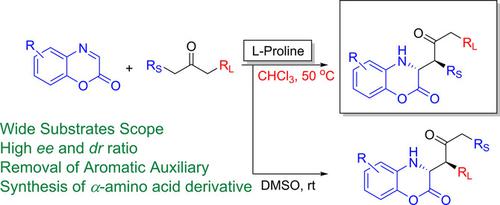
In this study, we have developed solvent‐controlled regioselective Mannich reaction of cyclicimines (benzoxazinones) with various ketones by using ʟ‐proline as catalyst to afford Mannich products in high yields with excellent enantio‐ and diastereoselectivity. The unsymmetrical ketones produced the linear isomer as the major product in chloroform solvent. On contrary, using the DMSO solvent favoured the formation of branch isomer product with excellent enantio‐ and diastereoselectivity. The aromatic auxiliary was successfully removed and afforded N‐protected chiral amino acid derivative.
中文翻译:

酮与苯并恶嗪酮的有机催化和区域发散曼尼希反应
在这项研究中,我们通过使用solvent-脯氨酸作为催化剂,开发了溶剂控制的环亚胺(苯并恶嗪酮)与各种酮的区域选择性曼尼希反应,以高收率提供曼尼希产品,具有出色的对映和非对映选择性。在氯仿溶剂中,不对称酮产生的线性异构体为主要产物。相反,使用DMSO溶剂有利于形成具有出色对映和非对映选择性的支链异构体产物。成功去除了芳香助剂,并提供了N保护的手性氨基酸衍生物。
更新日期:2018-10-08
中文翻译:

酮与苯并恶嗪酮的有机催化和区域发散曼尼希反应
在这项研究中,我们通过使用solvent-脯氨酸作为催化剂,开发了溶剂控制的环亚胺(苯并恶嗪酮)与各种酮的区域选择性曼尼希反应,以高收率提供曼尼希产品,具有出色的对映和非对映选择性。在氯仿溶剂中,不对称酮产生的线性异构体为主要产物。相反,使用DMSO溶剂有利于形成具有出色对映和非对映选择性的支链异构体产物。成功去除了芳香助剂,并提供了N保护的手性氨基酸衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号