当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative coupling strategies for the synthesis of indole alkaloids
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2018-09-17 00:00:00 , DOI: 10.1039/c8cs00305j Karre Nagaraju 1, 2, 3, 4, 5 , Dawei Ma 1, 2, 3, 4, 5
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2018-09-17 00:00:00 , DOI: 10.1039/c8cs00305j Karre Nagaraju 1, 2, 3, 4, 5 , Dawei Ma 1, 2, 3, 4, 5
Affiliation
|
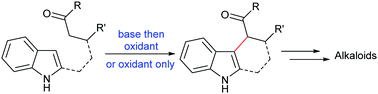
Direct bond formation between two C–H bonds is most challenging but imperative for efficient organic synthesis. Recently, significant progress has been made in direct functionalization of indole through oxidative coupling reactions with other nucleophiles such as enolates and phenols. Both intermolecular and intramolecular coupling reactions can be conducted under the action of base/oxidants or oxidants alone. Coupling typically occurs at the 3-position of indole moiety due to intrinsic nucleophilicity at this position. Coupling at the 2- or 4-position of the indole moiety has been observed for some special substrates. These coupling reactions provide powerful tools for quickly establishing the core structures of a number of indole alkaloids.
中文翻译:

吲哚生物碱合成的氧化偶联策略
在两个C–H键之间直接形成键是最具挑战性的,但对于有效的有机合成而言则势在必行。最近,通过与其他亲核试剂如烯醇酸酯和酚的氧化偶联反应,在吲哚的直接官能化方面已取得重大进展。分子间和分子内偶联反应都可以在碱/氧化剂或单独氧化剂的作用下进行。由于在该位置的固有亲核性,偶联通常发生在吲哚部分的3位。对于某些特殊的底物,已经观察到在吲哚部分的2-或4-位偶联。这些偶联反应为快速建立许多吲哚生物碱的核心结构提供了强大的工具。
更新日期:2018-09-17
中文翻译:

吲哚生物碱合成的氧化偶联策略
在两个C–H键之间直接形成键是最具挑战性的,但对于有效的有机合成而言则势在必行。最近,通过与其他亲核试剂如烯醇酸酯和酚的氧化偶联反应,在吲哚的直接官能化方面已取得重大进展。分子间和分子内偶联反应都可以在碱/氧化剂或单独氧化剂的作用下进行。由于在该位置的固有亲核性,偶联通常发生在吲哚部分的3位。对于某些特殊的底物,已经观察到在吲哚部分的2-或4-位偶联。这些偶联反应为快速建立许多吲哚生物碱的核心结构提供了强大的工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号