Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2018-09-17 , DOI: 10.1016/j.jinorgbio.2018.09.013 Valeria M. Nurchi , Guido Crisponi , Joanna I. Lachowicz , Maria de Guadalupe Jaraquemada-Pelaez , Clemente Bretti , Massimiliano Peana , Serenella Medici , Maria Antonietta Zoroddu
|
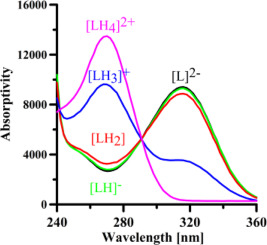
This paper presents an easy and low cost synthesis of chelating agents for potential medical and environmental applications, and the evaluation of the stability of their complexes with Fe3+, Al3+, Cu2+ and Zn2+. In the last years, we synthesized and characterized effective iron chelators based on two kojic acid units joined by different linkers in position 6. In this study, we preserved kojic acid (a cheap and non-toxic molecule) as the basic unit but joined the two kojic acid units through ethylene diamine, propylene diamine and butylene diamine by reacting them with the OH groups in position 2. The different anchoring position of the linker, as well as the linker length, can affect both protonation and complex formation equilibria. A thorough study of the protonation and complex formation equilibria of the three ligands toward the metal ions is presented based on combined potentiometric and spectroscopic studies, and 1H NMR. The obtained results allow remarking that the orientation of the oxygen atoms in the kojic acid units, related to the anchoring position of the linker, strongly affects the protonation constants, while the chelating ability is practically unaffected. The trivalent metal ions form stable complexes with a 2:3 metal to ligand stoichiometry through the oxygen donor atoms of the ligands, whereas divalent metal ions form binuclear complexes for which the nitrogen atoms from the linker might be involved in the coordination sphere. The stability of the complexes decreases with linker length, and the selectivity of the ligands toward metal ions grows in the order Zn2+ < Cu2+ < Al3+ < Fe3+.
中文翻译:

新型双羟基吡喃酮衍生物与Fe 3+,Al 3+,Cu 2+和Zn 2+的平衡研究
本文提出了一种容易和低成本合成的螯合剂,用于潜在的医学和环境应用,并评估了它们与Fe 3+,Al 3+,Cu 2+和Zn 2+的配合物的稳定性。。在过去的几年中,我们基于两个在位置6上不同接头连接的曲酸单元,合成和表征了有效的铁螯合剂。在这项研究中,我们保留了曲酸(一种廉价且无毒的分子)作为基本单元,但加入了两个乙二酸单元通过乙二胺,丙二胺和丁二胺与2位上的OH基反应而形成。连接基的不同锚固位置以及连接基长度会影响质子化和络合物形成平衡。基于电位和光谱学的综合研究,对三种配体对金属离子的质子化和复合物形成平衡进行了详尽的研究,其中11 H NMR。所得结果表明,曲酸单元中氧原子的取向与连接基的锚定位置有关,强烈影响质子化常数,而螯合能力实际上不受影响。三价金属离子通过配体的氧供体原子与金属与配体化学计量比为2:3形成稳定的络合物,而二价金属离子形成双核络合物,来自连接子的氮原子可能参与配位球。配合物的稳定性随连接体长度的增加而降低,并且配体对金属离子的选择性以Zn 2+ <Cu 2+ <Al 3+ <Fe 3+的顺序增长。











































 京公网安备 11010802027423号
京公网安备 11010802027423号