Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of the membrane-assembled retromer coat determined by cryo-electron tomography
Nature ( IF 50.5 ) Pub Date : 2018-09-01 , DOI: 10.1038/s41586-018-0526-z Oleksiy Kovtun 1, 2 , Natalya Leneva 3, 4 , Yury S Bykov 1, 2 , Nicholas Ariotti 3, 5 , Rohan D Teasdale 3, 6 , Miroslava Schaffer 7 , Benjamin D Engel 7 , David J Owen 4 , John A G Briggs 1, 2 , Brett M Collins 3
Nature ( IF 50.5 ) Pub Date : 2018-09-01 , DOI: 10.1038/s41586-018-0526-z Oleksiy Kovtun 1, 2 , Natalya Leneva 3, 4 , Yury S Bykov 1, 2 , Nicholas Ariotti 3, 5 , Rohan D Teasdale 3, 6 , Miroslava Schaffer 7 , Benjamin D Engel 7 , David J Owen 4 , John A G Briggs 1, 2 , Brett M Collins 3
Affiliation
|
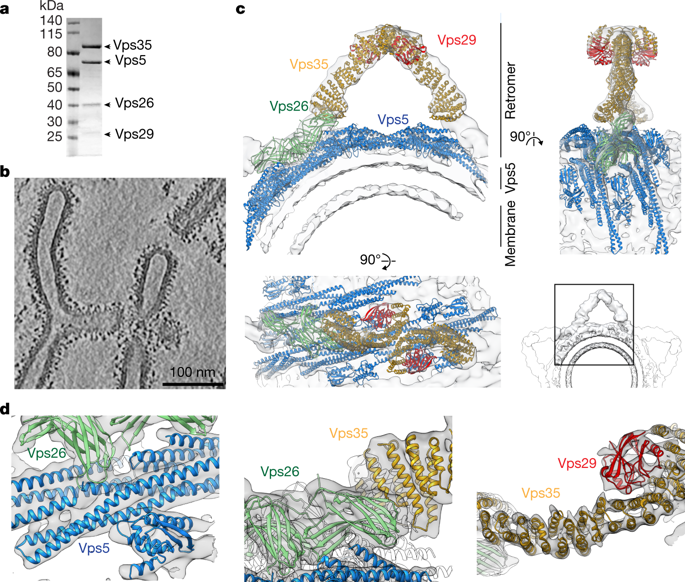
Eukaryotic cells traffic proteins and lipids between different compartments using protein-coated vesicles and tubules. The retromer complex is required to generate cargo-selective tubulovesicular carriers from endosomal membranes1–3. Conserved in eukaryotes, retromer controls the cellular localization and homeostasis of hundreds of transmembrane proteins, and its disruption is associated with major neurodegenerative disorders4–7. How retromer is assembled and how it is recruited to form coated tubules is not known. Here we describe the structure of the retromer complex (Vps26–Vps29–Vps35) assembled on membrane tubules with the bin/amphiphysin/rvs-domain-containing sorting nexin protein Vps5, using cryo-electron tomography and subtomogram averaging. This reveals a membrane-associated Vps5 array, from which arches of retromer extend away from the membrane surface. Vps35 forms the ‘legs’ of these arches, and Vps29 resides at the apex where it is free to interact with regulatory factors. The bases of the arches connect to each other and to Vps5 through Vps26, and the presence of the same arches on coated tubules within cells confirms their functional importance. Vps5 binds to Vps26 at a position analogous to the previously described cargo- and Snx3-binding site, which suggests the existence of distinct retromer-sorting nexin assemblies. The structure provides insight into the architecture of the coat and its mechanism of assembly, and suggests that retromer promotes tubule formation by directing the distribution of sorting nexin proteins on the membrane surface while providing a scaffold for regulatory-protein interactions.The retromer complex (the vacuolar protein sorting heterotrimer Vps26–Vps29–Vps35) has been resolved in association with membranes and the sorting nexin protein Vps5 using cryo-electron tomography.
中文翻译:

冷冻电子断层扫描确定的膜组装逆转录涂层的结构
真核细胞使用蛋白质包被的囊泡和小管在不同区室之间运输蛋白质和脂质。逆转录酶复合体需要从内体膜 1-3 产生货物选择性管泡载体。在真核生物中保守的逆转录酶控制着数百种跨膜蛋白的细胞定位和稳态,其破坏与主要的神经退行性疾病有关 4-7。逆转录酶是如何组装的以及它是如何被募集以形成涂层小管的尚不清楚。在这里,我们使用低温电子断层扫描和子断层图平均描述了逆转录酶复合体 (Vps26–Vps29–Vps35) 的结构,这些逆转录酶复合物 (Vps26–Vps29–Vps35) 组装在膜管上,其中包含 bin/amphiphysin/rvs 结构域的分选连接蛋白 Vps5。这揭示了一个膜相关的 Vps5 阵列,逆转录酶的拱门从膜表面延伸开来。Vps35 形成这些拱门的“腿”,而 Vps29 位于顶点,可以自由地与调节因子相互作用。拱门的底部相互连接并通过 Vps26 连接到 Vps5,细胞内涂层小管上存在相同的拱门证实了它们的功能重要性。Vps5 在类似于先前描述的货物和 Snx3 结合位点的位置与 Vps26 结合,这表明存在不同的逆转录酶分选连接蛋白组件。该结构提供了对外壳结构及其组装机制的深入了解,并表明逆转录酶通过指导分选连接蛋白在膜表面的分布来促进小管形成,同时为调节蛋白相互作用提供支架。
更新日期:2018-09-01
中文翻译:

冷冻电子断层扫描确定的膜组装逆转录涂层的结构
真核细胞使用蛋白质包被的囊泡和小管在不同区室之间运输蛋白质和脂质。逆转录酶复合体需要从内体膜 1-3 产生货物选择性管泡载体。在真核生物中保守的逆转录酶控制着数百种跨膜蛋白的细胞定位和稳态,其破坏与主要的神经退行性疾病有关 4-7。逆转录酶是如何组装的以及它是如何被募集以形成涂层小管的尚不清楚。在这里,我们使用低温电子断层扫描和子断层图平均描述了逆转录酶复合体 (Vps26–Vps29–Vps35) 的结构,这些逆转录酶复合物 (Vps26–Vps29–Vps35) 组装在膜管上,其中包含 bin/amphiphysin/rvs 结构域的分选连接蛋白 Vps5。这揭示了一个膜相关的 Vps5 阵列,逆转录酶的拱门从膜表面延伸开来。Vps35 形成这些拱门的“腿”,而 Vps29 位于顶点,可以自由地与调节因子相互作用。拱门的底部相互连接并通过 Vps26 连接到 Vps5,细胞内涂层小管上存在相同的拱门证实了它们的功能重要性。Vps5 在类似于先前描述的货物和 Snx3 结合位点的位置与 Vps26 结合,这表明存在不同的逆转录酶分选连接蛋白组件。该结构提供了对外壳结构及其组装机制的深入了解,并表明逆转录酶通过指导分选连接蛋白在膜表面的分布来促进小管形成,同时为调节蛋白相互作用提供支架。











































 京公网安备 11010802027423号
京公网安备 11010802027423号