当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparison of Reactivity and Enantioselectivity between Chiral Bimetallic Catalysts: Bismuth–Rhodium- and Dirhodium-Catalyzed Carbene Chemistry
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-09-14 00:00:00 , DOI: 10.1021/acscatal.8b03054 Zhi Ren 1 , Travis L. Sunderland 2 , Cecilia Tortoreto 1 , Tzuhsiung Yang 2 , John F. Berry 2 , Djamaladdin G. Musaev 3 , Huw M. L. Davies 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-09-14 00:00:00 , DOI: 10.1021/acscatal.8b03054 Zhi Ren 1 , Travis L. Sunderland 2 , Cecilia Tortoreto 1 , Tzuhsiung Yang 2 , John F. Berry 2 , Djamaladdin G. Musaev 3 , Huw M. L. Davies 1
Affiliation
|
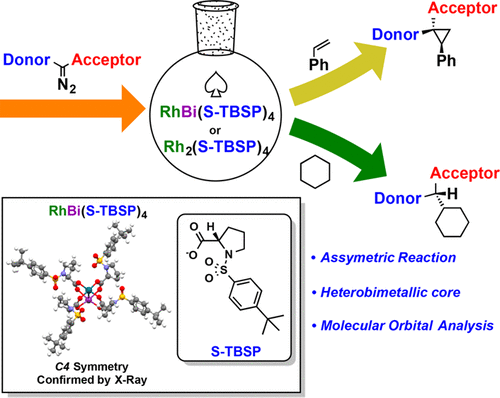
This paper describes the influence of replacement of one of the rhodium atoms by bismuth in a chiral dirhodium tetracarboxylate catalyst on asymmetric induction in the cyclopropanation and C–H functionalization chemistry of trichloroethyl aryldiazoacetates. The chiral ligand used in this study is S-tert-butylsulphonylprolinate (S-TBSP), which was used in the first highly enantioselective dirhodium tetracarboxylate catalyst. Even though the replacement of a Rh atom with a Bi atom has changed the electronic properties of the system, the RhBi complexes have several similarities to the corresponding Rh2 catalysts, with regard to their reactions with donor/acceptor carbenes. The asymmetric induction with RhBi(S-TBSP)4 is very similar to that achieved with Rh2(S-TBSP)4. The major differences between the two systems are the rates of reactions with the Rh2 complexes reacting much faster, and the scope of the C–H functionalization with Rh2 complexes capable of catalyzing reactions with a much wider range of substrates. An unexpected structural feature of the RhBi(S-TBSP)4 catalyst is the arrangement of the arylsulfonyl groups in the periphery of the catalyst, leading to a C4 symmetric structure.
中文翻译:

手性双金属催化剂之间的反应性和对映选择性的比较:铋-铑和二铑催化的碳烯化学
本文描述了在手性四羧酸四氢二铜铑催化剂中,铋取代一个铑原子对三氯乙基芳基重氮乙酸酯的环丙烷化和CH功能化反应中的不对称诱导的影响。在此研究中使用的手性配体是小号-叔-butylsulphonylprolinate(小号-TBSP),其在所述第一高度的对映选择性二铑催化剂四羧酸酯使用。即使用Bi原子代替Rh原子已改变了系统的电子性质,但RhBi配合物与相应的Rh 2催化剂在与供体/受体卡宾的反应方面仍具有一些相似性。RhBi(S-TBSP)4与用Rh 2(S -TBSP)4实现的非常相似。两个系统之间的主要区别是与铑反应的速率2络合物反应快得多,并与铑的C-H官能的范围2能够与更宽范围的底物催化反应的复合物。RhBi(S- TBSP)4催化剂的出乎意料的结构特征是芳基磺酰基在催化剂外围的排列,导致C 4对称结构。
更新日期:2018-09-14
中文翻译:

手性双金属催化剂之间的反应性和对映选择性的比较:铋-铑和二铑催化的碳烯化学
本文描述了在手性四羧酸四氢二铜铑催化剂中,铋取代一个铑原子对三氯乙基芳基重氮乙酸酯的环丙烷化和CH功能化反应中的不对称诱导的影响。在此研究中使用的手性配体是小号-叔-butylsulphonylprolinate(小号-TBSP),其在所述第一高度的对映选择性二铑催化剂四羧酸酯使用。即使用Bi原子代替Rh原子已改变了系统的电子性质,但RhBi配合物与相应的Rh 2催化剂在与供体/受体卡宾的反应方面仍具有一些相似性。RhBi(S-TBSP)4与用Rh 2(S -TBSP)4实现的非常相似。两个系统之间的主要区别是与铑反应的速率2络合物反应快得多,并与铑的C-H官能的范围2能够与更宽范围的底物催化反应的复合物。RhBi(S- TBSP)4催化剂的出乎意料的结构特征是芳基磺酰基在催化剂外围的排列,导致C 4对称结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号