当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering a Next-Generation Glycoconjugate-Like Streptococcus pneumoniae Vaccine
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2018-09-04 00:00:00 , DOI: 10.1021/acsinfecdis.8b00100 Andrew B Hill 1, 2 , Marie Beitelshees 1, 2 , Roozbeh Nayerhoda 3 , Blaine A Pfeifer 2, 3 , Charles H Jones 1
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2018-09-04 00:00:00 , DOI: 10.1021/acsinfecdis.8b00100 Andrew B Hill 1, 2 , Marie Beitelshees 1, 2 , Roozbeh Nayerhoda 3 , Blaine A Pfeifer 2, 3 , Charles H Jones 1
Affiliation
|
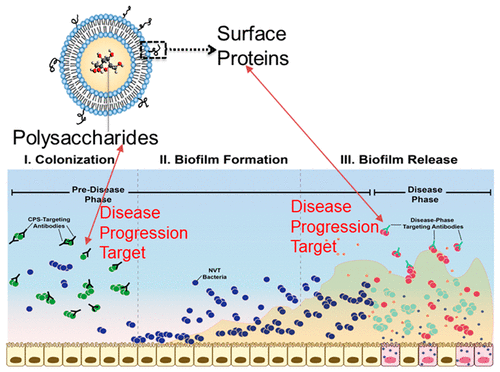
We detail the development of a next-generation Streptococcus pneumoniae liposomal encapsulation of polysaccharides (LEPS) vaccine, with design characteristics geared toward best-in-class efficacy. The first generation LEPS vaccine, which contained 20 encapsulated pneumococcal capsular polysaccharides (CPSs) and two surface-displayed virulence-associated proteins (GlpO and PncO), enabling prophylactic potency against 70+ serotypes of Streptococcus pneumoniae (the causative agent of pneumococcal disease), was rationally redesigned for advanced clinical readiness and best-in-class coverage. In doing so, the virulent-specific GlpO protein antigen was removed from the final formulation due to off-target immunogenicity toward bacterial species within the human microbiome, while directed protection was maintained by increasing the dose of PncO from 17 to 68 μg. LEPS formulation parameters also readily facilitated an increase in CPS valency (to a total of 24) and systematic variation in protein–liposome attachment mechanisms in anticipation of clinical translation. An additional safety assessment study demonstrated that LEPS does not exhibit appreciable toxicological effects even when administered at ten times the effective dose. In summary, this new design offers the broadest, safest, and most-complete protection while maintaining desirable glycoconjugate-like features, positioning the LEPS vaccine platform for clinical success and a global health impact.
中文翻译:

设计下一代糖复合物样肺炎链球菌疫苗
我们详细介绍了下一代肺炎链球菌多糖脂质体包封 (LEPS) 疫苗的开发,其设计特点旨在实现一流的疗效。第一代 LEPS 疫苗,包含 20 种封装的肺炎球菌荚膜多糖 (CPS) 和两种表面展示的毒力相关蛋白(GlpO 和 PncO),具有针对 70 多种血清型肺炎链球菌的预防效力(肺炎球菌疾病的病原体),经过合理重新设计,以实现先进的临床准备和一流的覆盖范围。在此过程中,由于对人类微生物组内细菌物种的脱靶免疫原性,从最终制剂中去除了强毒特异性 GlpO 蛋白抗原,同时通过将 PncO 的剂量从 17 μg 增加到 68 μg 来维持定向保护。LEPS 配方参数也很容易促进 CPS 效价的增加(达到总计 24)和蛋白质-脂质体附着机制的系统变化,以预期临床转化。一项额外的安全评估研究表明,即使以有效剂量的十倍给药,LEPS 也不会表现出明显的毒理学效应。总之,这种新设计提供了最广泛、最安全、
更新日期:2018-09-04
中文翻译:

设计下一代糖复合物样肺炎链球菌疫苗
我们详细介绍了下一代肺炎链球菌多糖脂质体包封 (LEPS) 疫苗的开发,其设计特点旨在实现一流的疗效。第一代 LEPS 疫苗,包含 20 种封装的肺炎球菌荚膜多糖 (CPS) 和两种表面展示的毒力相关蛋白(GlpO 和 PncO),具有针对 70 多种血清型肺炎链球菌的预防效力(肺炎球菌疾病的病原体),经过合理重新设计,以实现先进的临床准备和一流的覆盖范围。在此过程中,由于对人类微生物组内细菌物种的脱靶免疫原性,从最终制剂中去除了强毒特异性 GlpO 蛋白抗原,同时通过将 PncO 的剂量从 17 μg 增加到 68 μg 来维持定向保护。LEPS 配方参数也很容易促进 CPS 效价的增加(达到总计 24)和蛋白质-脂质体附着机制的系统变化,以预期临床转化。一项额外的安全评估研究表明,即使以有效剂量的十倍给药,LEPS 也不会表现出明显的毒理学效应。总之,这种新设计提供了最广泛、最安全、









































 京公网安备 11010802027423号
京公网安备 11010802027423号