当前位置:
X-MOL 学术
›
ACS Comb. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ring-Opening of Indoles: An Unconventional Route for the Transformation of Indoles to 1H-Pyrazoles Using Lewis Acid
ACS Combinatorial Science Pub Date : 2018-09-10 00:00:00 , DOI: 10.1021/acscombsci.8b00071 Subhankar Panda 1 , Nirmalya Pradhan 1 , Debasis Manna 1
ACS Combinatorial Science Pub Date : 2018-09-10 00:00:00 , DOI: 10.1021/acscombsci.8b00071 Subhankar Panda 1 , Nirmalya Pradhan 1 , Debasis Manna 1
Affiliation
|
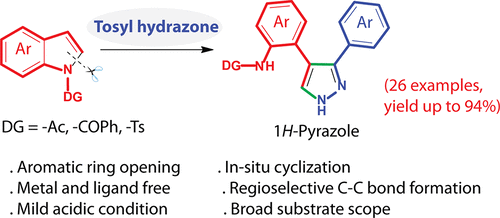
An unusual transformation of indoles to pyrazoles via an aromatic ring-opening strategy has been developed. The salient feature of this strategy involves the C2–N1 bond opening and concomitant cyclization reaction of the C2═C3 bond of the indole moiety with the tosylhydrazone, which proceeds under transition-metal and ligand free conditions. This ring-opening functionalization of indoles provides a wide scope of differently substituted pyrazoles.
中文翻译:

吲哚的开环:使用路易斯酸将吲哚转化为1 H-吡唑的非常规途径
已经开发出通过芳族开环策略将吲哚不寻常地转化为吡唑。该策略的显着特征涉及C2–N1键的打开以及吲哚部分的C2═C3键与甲苯磺酰zone的环化反应,该反应在无过渡金属和无配体的条件下进行。吲哚的这种开环官能化提供了范围广泛的不同取代的吡唑。
更新日期:2018-09-10
中文翻译:

吲哚的开环:使用路易斯酸将吲哚转化为1 H-吡唑的非常规途径
已经开发出通过芳族开环策略将吲哚不寻常地转化为吡唑。该策略的显着特征涉及C2–N1键的打开以及吲哚部分的C2═C3键与甲苯磺酰zone的环化反应,该反应在无过渡金属和无配体的条件下进行。吲哚的这种开环官能化提供了范围广泛的不同取代的吡唑。











































 京公网安备 11010802027423号
京公网安备 11010802027423号