Chemistry and Physics of Lipids ( IF 3.4 ) Pub Date : 2018-09-14 , DOI: 10.1016/j.chemphyslip.2018.09.006 Jasmeet Singh 1 , Miroslav Peric 1
|
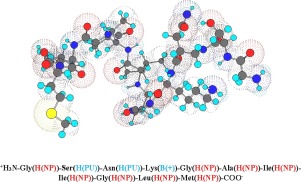
The interactions of the Alzheimer’s β-amyloid peptide, Aβ(25–35), with 18:1 (Δ9-Cis) PC 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), L-α-phosphatidylcholine (EPC), 1,2-dioleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (sodium salt) (DOPG), and L-α-phosphatidylglycerol (EPG) phospholipid vesicles with and without cholesterol (Ch) are studied by the nitroxide spin probe electron paramagnetic resonance (EPR) method. Two nitroxide spin probes, 2,2,6,6-tetramethyl-piperidin-1-oxyl-4-yl hexadecanoate (TP, TEMPO-Palmitate) and 2-Ethyl-2-(15-methoxy-15-oxopentadecyl)-4,4-dimethyl-3-oxazolidinyloxy (16-DSE), are utilized in the study. TEMPO-Palmitate has the reporting EPR moiety located at the top of this spin probe, while 16-DSE has the reporting EPR moiety located at the tail of the spin probe. These two probes enable us to sample the surface and the middle of the phospholipid bilayer, respectively. All EPR measurements are done above the melting points of all four phospholipids when the bilayer is in the liquid crystal phase, the physiologically relevant phase. Due to non-linear spectral line fitting, the EPR spectral parameters are extracted with high precision. The results show that there are two populations of Aβ(25–35) and that one of them is located in the hydrophobic phospholipid layer below the hydrophilic headgroup region. The second population appears to be weakly coupled to the surface of the bilayer. Both hydrophobic and electrostatic interactions affect the insertion of Aβ(25–35) in the bilayer. Also, there is strong evidence for an interaction between cholesterol and Aβ(25–35), which affects the dielectric and dynamic properties of the bilayer.
中文翻译:

β淀粉样蛋白-Aβ(25-35)-肽与带有或不带有胆固醇的两性离子和带负电荷的囊泡的相互作用。
阿尔茨海默氏症的β-淀粉样肽Aβ(25-35)与18:1(Δ9-Cis)PC 1,2-二油酰基-sn的相互作用-甘油-3-磷酸胆碱(DOPC),L-α-磷脂酰胆碱(EPC),1,2-二油酰基-sn-甘油3-磷酸-(1'-rac-甘油)(钠盐)(DOPG)和通过一氧化氮自旋探针电子顺磁共振(EPR)方法研究了具有和不具有胆固醇(Ch)的L-α-磷脂酰甘油(EPG)磷脂囊泡。两个氮氧化物自旋探针,2,2,6,6-四甲基哌啶-1-氧基-4-基十六烷酸酯(TP,TEMPO-棕榈酸酯)和2-乙基-2-(15-甲氧基-15-氧十五烷基)-4在研究中使用了4-4-二甲基-3-恶唑烷二氧基(16-DSE)。TEMPO-Palmitate的报告EPR部分位于此旋转探针的顶部,而16-DSE的报告EPR部分位于该旋转探针的尾部。这两个探针使我们能够分别采样磷脂双层的表面和中间层。当双层处于液晶相(生理学相关相)时,所有EPR测量均在所有四个磷脂的熔点以上进行。由于非线性光谱线拟合,因此可以高精度提取EPR光谱参数。结果表明,有两个Aβ(25–35)群体,其中一个位于亲水性头基区域下方的疏水性磷脂层中。第二人口似乎弱耦合到双层的表面。疏水和静电相互作用均会影响双层中Aβ(25–35)的插入。同样,有强有力的证据表明胆固醇和Aβ(25-35)之间存在相互作用,这会影响双层的介电和动态特性。生理相关阶段。由于非线性光谱线拟合,因此可以高精度提取EPR光谱参数。结果表明,有两个Aβ(25–35)群体,其中一个位于亲水性头基区域下方的疏水性磷脂层中。第二人口似乎弱耦合到双层的表面。疏水和静电相互作用均会影响双层中Aβ(25–35)的插入。同样,有强有力的证据表明胆固醇和Aβ(25-35)之间存在相互作用,这会影响双层的介电和动态特性。生理相关阶段。由于非线性光谱线拟合,因此可以高精度提取EPR光谱参数。结果表明,有两个Aβ(25-35)群体,其中一个位于亲水性头基区域下方的疏水性磷脂层中。第二人口似乎弱耦合到双层的表面。疏水和静电相互作用均会影响双层中Aβ(25–35)的插入。同样,有强有力的证据表明胆固醇和Aβ(25-35)之间存在相互作用,这会影响双层的介电和动态特性。结果表明,有两个Aβ(25-35)群体,其中一个位于亲水性头基区域下方的疏水性磷脂层中。第二人口似乎弱耦合到双层的表面。疏水和静电相互作用均会影响双层中Aβ(25–35)的插入。同样,有强有力的证据表明胆固醇和Aβ(25-35)之间存在相互作用,这会影响双层的介电和动态特性。结果表明,有两个Aβ(25–35)群体,其中一个位于亲水性头基区域下方的疏水性磷脂层中。第二人口似乎弱耦合到双层的表面。疏水和静电相互作用均会影响双层中Aβ(25–35)的插入。同样,有强有力的证据表明胆固醇和Aβ(25-35)之间存在相互作用,这会影响双层的介电和动态特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号