Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ionic Transport and Speciation of Lithium Salts in Glymes: Experimental and Theoretical Results for Electrolytes of Interest for Lithium–Air Batteries
ACS Omega ( IF 3.7 ) Pub Date : 2018-09-14 00:00:00 , DOI: 10.1021/acsomega.8b01443 Gabriela Horwitz 1, 2 , Matías Factorovich 1 , Javier Rodriguez 2, 3, 4 , Daniel Laria 1, 2, 3 , Horacio R Corti 1, 2, 3
ACS Omega ( IF 3.7 ) Pub Date : 2018-09-14 00:00:00 , DOI: 10.1021/acsomega.8b01443 Gabriela Horwitz 1, 2 , Matías Factorovich 1 , Javier Rodriguez 2, 3, 4 , Daniel Laria 1, 2, 3 , Horacio R Corti 1, 2, 3
Affiliation
|
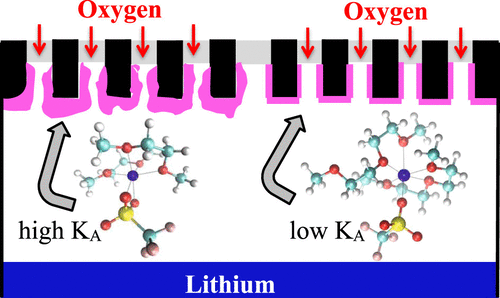
Glycol ethers, or glymes, have been recognized as good candidates as solvents for lithium–air batteries because they exhibit relatively good stability in the presence of superoxide radicals. Diglyme (bis(2-methoxy-ethyl)ether), in spite of its low donor number, has been found to promote the solution mechanism for the formation of Li2O2 during the discharge reaction, leading to large deposits, that is, high capacities. It has been suggested that lithium salt association in these types of solvents could be responsible for this behavior. Thus, the knowledge of the speciation and transport behavior of lithium salts in these types of solvents is relevant for the optimization of the lithium–air battery performance. In this work, a comprehensive study of lithium trifluoromethanesulfonate (LiTf) and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) in 1,2-di-methoxyethane (DME) and diglyme, over a wide range of concentrations, have been performed. Consistent ion pairs and triplet ions formation constants have been obtained by resorting to well-known equations that describe the concentration dependence of the molar conductivities in highly associated electrolytes, and we found that the system LiTf/DME would be the best to promote bulky Li2O2 deposits. Unexpected differences are observed for the association constants of LiTf and, to a lesser extent, for LiTFSI, in DME and diglyme, whose dielectric constants are similar. Molecular dynamics (MD) simulations allowed us to rationalize these differences in terms of the competing interactions of the O-sites of the ethers and the SOx groups of the corresponding anions with Li+ ion. The limiting Li+ diffusivity derived from the fractional Walden rule agrees quite well with those obtained from MD simulations, when solvent viscosity is conveniently rescaled.
中文翻译:

甘醇二甲醚中锂盐的离子传输和形态:锂空气电池相关电解质的实验和理论结果
乙二醇醚或甘醇二甲醚已被认为是锂空气电池溶剂的良好候选者,因为它们在超氧自由基存在下表现出相对良好的稳定性。二甘醇二甲醚(双(2-甲氧基乙基)醚)尽管供体数量较低,但已被发现可以促进放电反应过程中形成Li 2 O 2的溶液机制,从而导致大量沉积物,即高容量。有人认为,这些类型的溶剂中的锂盐缔合可能是造成这种行为的原因。因此,了解锂盐在这些类型溶剂中的形态和传输行为与锂空气电池性能的优化相关。在这项工作中,对 1,2-二甲氧基乙烷 (DME) 和二甘醇二甲醚中的三氟甲磺酸锂 (LiTf) 和双(三氟甲磺酰基)亚胺锂 (LiTFSI) 在各种浓度下进行了全面研究。通过采用描述高度缔合电解质中摩尔电导率的浓度依赖性的众所周知的方程,我们获得了一致的离子对和三重态离子形成常数,并且我们发现LiTf/DME系统将是促进大体积Li 2的最佳选择O 2沉积物。在介电常数相似的 DME 和二甘醇二甲醚中,LiTf 的缔合常数以及较小程度的 LiTFSI 的缔合常数出现了意想不到的差异。分子动力学 (MD) 模拟使我们能够根据醚的 O 位点和相应阴离子的 SO x基团与 Li +离子的竞争相互作用来合理化这些差异。 当溶剂粘度方便地重新调整时,从分数瓦尔登规则得出的限制 Li +扩散率与从 MD 模拟获得的结果非常一致。
更新日期:2018-09-14
中文翻译:

甘醇二甲醚中锂盐的离子传输和形态:锂空气电池相关电解质的实验和理论结果
乙二醇醚或甘醇二甲醚已被认为是锂空气电池溶剂的良好候选者,因为它们在超氧自由基存在下表现出相对良好的稳定性。二甘醇二甲醚(双(2-甲氧基乙基)醚)尽管供体数量较低,但已被发现可以促进放电反应过程中形成Li 2 O 2的溶液机制,从而导致大量沉积物,即高容量。有人认为,这些类型的溶剂中的锂盐缔合可能是造成这种行为的原因。因此,了解锂盐在这些类型溶剂中的形态和传输行为与锂空气电池性能的优化相关。在这项工作中,对 1,2-二甲氧基乙烷 (DME) 和二甘醇二甲醚中的三氟甲磺酸锂 (LiTf) 和双(三氟甲磺酰基)亚胺锂 (LiTFSI) 在各种浓度下进行了全面研究。通过采用描述高度缔合电解质中摩尔电导率的浓度依赖性的众所周知的方程,我们获得了一致的离子对和三重态离子形成常数,并且我们发现LiTf/DME系统将是促进大体积Li 2的最佳选择O 2沉积物。在介电常数相似的 DME 和二甘醇二甲醚中,LiTf 的缔合常数以及较小程度的 LiTFSI 的缔合常数出现了意想不到的差异。分子动力学 (MD) 模拟使我们能够根据醚的 O 位点和相应阴离子的 SO x基团与 Li +离子的竞争相互作用来合理化这些差异。 当溶剂粘度方便地重新调整时,从分数瓦尔登规则得出的限制 Li +扩散率与从 MD 模拟获得的结果非常一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号