Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-09-15 , DOI: 10.1016/j.actbio.2018.09.016 Linxiao Wu , Jatinder Virdee , Elizabeth Maughan , Arnold Darbyshire , Gavin Jell , Marilena Loizidou , Mark Emberton , Peter Butler , Ashley Howkins , Alan Reynolds , Ian W. Boyd , Martin Birchall , Wenhui Song
|
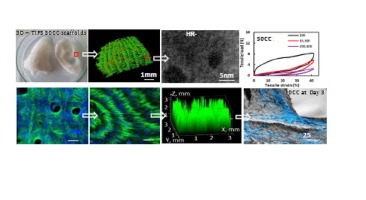
Cell and tissue stiffness is an important biomechanical signalling parameter for dynamic biological processes; responsive polymeric materials conferring responsive functionality are therefore appealing for in vivo implants. We have developed thermoresponsive poly(urea-urethane) nanohybrid scaffolds with ‘stiffness memory’ through a versatile 3D printing-guided thermally induced phase separation (3D-TIPS) technique. 3D-TIPS, a combination of 3D printing with phase separation, allows uniform phase-separation and phase transition of the polymer solution at a large interface of network within the printed sacrificial preform, leading to the creation of full-scale scaffolds with bespoke anatomical complex geometry. A wide range of hyperelastic mechanical properties of the soft elastomer scaffolds with interconnected pores at multi-scale, controlled porosity and crystallinity have been manufactured, not previously achievable via direct printing techniques or phase-separation alone. Semi-crystalline polymeric reverse self-assembly to a ground-stated quasi-random nanophase structure, throughout a hierarchical structure of internal pores, contributes to gradual stiffness relaxation during in vitro cell culture with minimal changes to shape. This ‘stiffness memory’ provides initial mechanical support to surrounding tissues before gradually softening to a better mechanical match, raising hopes for personalized and biologically responsive soft tissue implants which promote human fibroblast cells growth as model and potential scaffold tissue integration.
Statement of Significance
Biological processes are dynamic in nature, however current medical implants are often stronger and stiffer than the surrounding tissue, with little adaptability in response to biological and physical stimuli. This work has contributed to the development of a range of thermoresponsive nanohybrid elastomer scaffolds, with tuneable stiffness and hierarchically interconnected porous structure, manufactured by a versatile indirect 3D printing technique. For the first time, stiffness memory of the scaffold was observed to be driven by phase transition and a reverse self-assembly from a semicrystalline phase to a quasi-random nanostructured rubber phase. Early insight into cell response during the stiffness relaxation of the scaffolds in vitro holds promise for personalized biologically responsive soft implants.
中文翻译:

通过间接3D打印为生物响应性软植入物生成的刚度记忆纳米混合支架
细胞和组织的硬度是动态生物过程中重要的生物力学信号参数。因此,赋予响应性功能的响应性聚合材料在体内很有吸引力植入物。我们通过通用的3D打印引导热诱导相分离(3D-TIPS)技术开发了具有“刚度记忆”的热响应性聚(脲-氨基甲酸酯)纳米混合支架。3D-TIPS是3D打印与相分离的结合,可以在打印的牺牲型坯内的较大网络界面处实现聚合物溶液的均匀相分离和相变,从而产生具有定制解剖结构的全尺寸支架几何学。具有多尺度的相互连接的孔的软弹性体支架的宽范围的超弹性机械性能已经被制造出来,这是以前不能通过直接印刷技术或单独的相分离来实现的,这些孔隙率和结晶度是受控的。体外细胞培养,形状几乎没有变化。这种“刚度记忆”在逐渐变软以达到更好的机械匹配之前,为周围的组织提供了最初的机械支撑,这为个性化和具有生物学响应性的软组织植入物带来了希望,这些软组织植入物可以促进人类成纤维细胞的生长并成为潜在的支架组织整合体。
重要声明
生物过程本质上是动态的,但是当前的医疗植入物通常比周围的组织更坚固,对生物和物理刺激的适应性很小。这项工作推动了一系列热响应性纳米杂化弹性体支架的开发,这些支架具有可调节的刚度和通过各种间接3D打印技术制造的层次结构互连的多孔结构。首次观察到支架的刚度记忆是由相变和从半结晶相到准随机纳米结构橡胶相的反向自组装驱动的。对支架体外刚度松弛过程中细胞反应的早期了解为个性化生物反应性软植入物提供了希望。











































 京公网安备 11010802027423号
京公网安备 11010802027423号