当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Intramolecular alkene hydroamination and degradation of amidines: divergent behavior of rare earth metal amidinate intermediates†‡
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-09-14 00:00:00 , DOI: 10.1039/c8cy01481g Dexing Zhang 1, 2, 3, 4, 5 , Ruiting Liu 1, 2, 3, 4, 5 , Xigeng Zhou 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-09-14 00:00:00 , DOI: 10.1039/c8cy01481g Dexing Zhang 1, 2, 3, 4, 5 , Ruiting Liu 1, 2, 3, 4, 5 , Xigeng Zhou 1, 2, 3, 4, 5
Affiliation
|
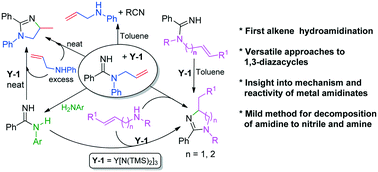
Direct N–H addition of amidines to alkenes is a highly valuable but challenging transformation that remains elusive. Now, the intramolecular hydroamidination of N-alkenylamidines is achieved by using a rare earth catalyst, which provides an efficient and atom-economical approach for substituted imidazolines and tetrahydropyrimidines. Moreover, a mild and efficient method for the catalytic degradation of amidines to give amines and nitriles is also developed. Additionally, amidine reconstruction followed by an intramolecular alkene hydroamidination strategy for the synthesis of substituted imidazolines and tetrahydropyrimidines from secondary enamines and inactive amidines has also been established, which may circumvent the need for some unavailable starting materials. The mechanistic studies prove that these reactions proceed via a key lanthanide amidinate intermediate that can undergo substrate- and amine-controlled chemodivergent transformations: intramolecular alkene insertion, nitrile extrusion, amidinate reconstruction, or a combination of the reactions. The results presented here not only demonstrate the synthetic potential and versatility of alkene hydroamidination with substrates, but also provide a good insight into the factors that promote or deter the hydroamidination of alkenes.
中文翻译:

分子内烯烃加氢胺化和degradation的降解:稀土金属a酰胺中间体的不同行为† ‡
将N-H直接直接添加到烯烃中是非常有价值的,但仍具有挑战性,目前仍难以捉摸。现在,N的分子内加氢酰胺化-烯基am是通过使用稀土催化剂获得的,稀土催化剂为取代的咪唑啉和四氢嘧啶提供了有效且原子经济的方法。此外,还开发了一种温和而有效的方法,用于催化将catalytic催化降解为胺和腈。另外,还建立了am重建,接着是分子内烯烃加氢酰胺化策略,用于由仲烯胺和非活性am合成取代的咪唑啉和四氢嘧啶,这可能避免了对某些无法获得的起始原料的需求。机理研究证明,这些反应通过可以经受底物和胺控制的化学发散转化的关键镧系a酰胺中间体:分子内烯烃插入,腈挤压,a酰胺重构或反应的组合。此处提供的结果不仅证明了烯烃与底物进行加氢酰胺化的合成潜力和多功能性,而且还对促进或阻止烯烃加氢酰胺化的因素提供了很好的见识。
更新日期:2018-09-14
中文翻译:

分子内烯烃加氢胺化和degradation的降解:稀土金属a酰胺中间体的不同行为† ‡
将N-H直接直接添加到烯烃中是非常有价值的,但仍具有挑战性,目前仍难以捉摸。现在,N的分子内加氢酰胺化-烯基am是通过使用稀土催化剂获得的,稀土催化剂为取代的咪唑啉和四氢嘧啶提供了有效且原子经济的方法。此外,还开发了一种温和而有效的方法,用于催化将catalytic催化降解为胺和腈。另外,还建立了am重建,接着是分子内烯烃加氢酰胺化策略,用于由仲烯胺和非活性am合成取代的咪唑啉和四氢嘧啶,这可能避免了对某些无法获得的起始原料的需求。机理研究证明,这些反应通过可以经受底物和胺控制的化学发散转化的关键镧系a酰胺中间体:分子内烯烃插入,腈挤压,a酰胺重构或反应的组合。此处提供的结果不仅证明了烯烃与底物进行加氢酰胺化的合成潜力和多功能性,而且还对促进或阻止烯烃加氢酰胺化的因素提供了很好的见识。



























 京公网安备 11010802027423号
京公网安备 11010802027423号