当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A bench stable formal Cu(iii) N-heterocyclic carbene accessible from simple copper(ii) acetate†‡
Chemical Science ( IF 7.6 ) Pub Date : 2018-09-14 00:00:00 , DOI: 10.1039/c8sc01834k Zohreh S Ghavami 1 , Markus R Anneser 2 , Felix Kaiser 2 , Philipp J Altmann 2 , Benjamin J Hofmann 2 , Jonas F Schlagintweit 2 , Gholamhossein Grivani 1 , Fritz E Kühn 2
Chemical Science ( IF 7.6 ) Pub Date : 2018-09-14 00:00:00 , DOI: 10.1039/c8sc01834k Zohreh S Ghavami 1 , Markus R Anneser 2 , Felix Kaiser 2 , Philipp J Altmann 2 , Benjamin J Hofmann 2 , Jonas F Schlagintweit 2 , Gholamhossein Grivani 1 , Fritz E Kühn 2
Affiliation
|
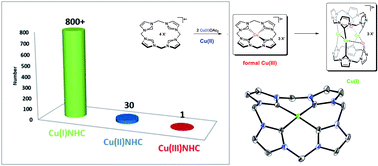
For years, Cu(III)NHCs have been proposed as active intermediates in Cu(I)NHC catalyzed reactions, yielding the desired products by reductive elimination, but until today, no one has ever reported the characterisation of such a compound. When working on the synthesis of biomimetic transition metal (NHC) complexes and their application in homogeneous catalysis, we recently found a highly unusual reactivity for Cu(II) acetate in the presence of a particular cyclic tetra(NHC) ligand. Therein, the formation of the first stable CuNHC compound, displaying Cu in the formal oxidation state +III, by simple disproportionation of Cu(II) acetate in dimethyl sulfoxide (DMSO) was observed. At elevated temperatures selective mono-oxidation of the NHC ligand occurs, even under anaerobic conditions. Acetate was identified as the origin of the oxygen atom by 18O-labelling experiments. The remarkably high stability of the title compound was furthermore proven electrochemically by cyclic voltammetry. An in-depth investigation of its reactivity revealed the involvement of four additional compounds. Three of them could be isolated and characterised by 1H/13C-NMR, single crystal XRD, mass spectrometry and elemental analysis. The fourth, a Cu(I)NHC intermediate, formed by formal reductive elimination from the Cu(NHC)3+ compound, was characterised in situ by 1H/13C-NMR and computational methods.
中文翻译:

可从简单的醋酸铜 (ii) 获得的台式稳定的形式 Cu(iii) N-杂环卡宾†‡
多年来,Cu( III )NHCs 一直被提议作为 Cu( I )NHC 催化反应的活性中间体,通过还原消除产生所需的产物,但直到今天,还没有人报道过这种化合物的表征。在研究仿生过渡金属 (NHC) 配合物的合成及其在均相催化中的应用时,我们最近发现在特定的环状四 (NHC) 配体存在下,乙酸铜 ( II ) 具有极不寻常的反应性。其中,第一个稳定的 CuNHC 化合物的形成,通过简单的 Cu( II) 在二甲亚砜 (DMSO) 中观察到乙酸盐。在高温下,即使在厌氧条件下,也会发生 NHC 配体的选择性单氧化。通过18 个O 标记实验,乙酸盐被确定为氧原子的来源。通过循环伏安法进一步证明了标题化合物的显着高稳定性。对其反应性的深入研究揭示了另外四种化合物的参与。其中三种可以通过1 H/ 13 C-NMR、单晶XRD、质谱和元素分析进行分离和表征。第四种,Cu( I )NHC 中间体,由 Cu(NHC) 3+化合物通过形式还原消除形成,其特征在于通过1 H/ 13 C-NMR和计算方法原位。
更新日期:2018-09-14
中文翻译:

可从简单的醋酸铜 (ii) 获得的台式稳定的形式 Cu(iii) N-杂环卡宾†‡
多年来,Cu( III )NHCs 一直被提议作为 Cu( I )NHC 催化反应的活性中间体,通过还原消除产生所需的产物,但直到今天,还没有人报道过这种化合物的表征。在研究仿生过渡金属 (NHC) 配合物的合成及其在均相催化中的应用时,我们最近发现在特定的环状四 (NHC) 配体存在下,乙酸铜 ( II ) 具有极不寻常的反应性。其中,第一个稳定的 CuNHC 化合物的形成,通过简单的 Cu( II) 在二甲亚砜 (DMSO) 中观察到乙酸盐。在高温下,即使在厌氧条件下,也会发生 NHC 配体的选择性单氧化。通过18 个O 标记实验,乙酸盐被确定为氧原子的来源。通过循环伏安法进一步证明了标题化合物的显着高稳定性。对其反应性的深入研究揭示了另外四种化合物的参与。其中三种可以通过1 H/ 13 C-NMR、单晶XRD、质谱和元素分析进行分离和表征。第四种,Cu( I )NHC 中间体,由 Cu(NHC) 3+化合物通过形式还原消除形成,其特征在于通过1 H/ 13 C-NMR和计算方法原位。











































 京公网安备 11010802027423号
京公网安备 11010802027423号