Electrochemistry Communications ( IF 4.7 ) Pub Date : 2018-09-13 , DOI: 10.1016/j.elecom.2018.09.007 Wei Zhou 1, 2 , Xiaoxiao Meng 1 , Ljiljana Rajic 2 , Yunfei Xue 2 , Shuai Chen 1 , Yani Ding 1 , Kaikai Kou 1 , Yan Wang 1 , Jihui Gao 1 , Yukun Qin 1 , Akram N Alshawabkeh 2
|
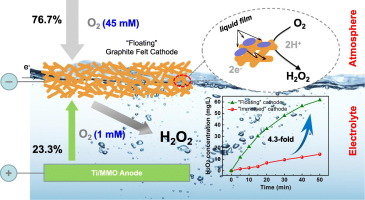
The performance of the Electro-Fenton (EF) process for contaminant degradation depends on the rate of H2O2 production at the cathode via 2-electron dissolved O2 reduction. However, the low solubility of O2 (≈1 × 10−3 mol dm−3) limits H2O2 production. Herein, a novel and practical strategy that enables the synergistic utilization of O2 from the bulk electrolyte and ambient air for efficient H2O2 production is proposed. Compared with a conventional “submerged” cathode, the H2O2 concentration obtained using the “floating” cathode is 4.3 and 1.5 times higher using porous graphite felt (GF) and reticulated vitreous carbon (RVC) foam electrodes, respectively. This surprising enhancement results from the formation of a three-phase interface inside the porous cathode, where the O2 from ambient air is also utilized for H2O2 production. The contribution of O2 from ambient air varies depending on the cathode material and is calculated to be 76.7% for the GF cathode and 35.6% for the RVC foam cathode. The effects of pH, current, and mixing on H2O2 production are evaluated. Finally, the EF process enhanced by the “floating” cathode degraded 78.3% of the anti-inflammatory drug ibuprofen after 120 min compared to only 25.4% using a conventional “submerged” electrode, without any increase in the cost.
中文翻译:

用于高效 H2O2 发电的“浮动”阴极,应用于降解布洛芬作为模型污染物
Electro-Fenton (EF) 工艺降解污染物的性能取决于阴极通过 2 电子溶解 O 2还原产生 H 2 O 2的速率。然而,O 2的低溶解度(≈1×10 -3 mol dm -3 )限制了H 2 O 2 的产生。在此,提出了一种新颖且实用的策略,该策略能够协同利用来自本体电解质和环境空气的O 2来高效生产H 2 O 2 。与传统的“浸入式”阴极相比,使用多孔石墨毡(GF)和网状玻璃碳(RVC)泡沫电极的“浮动”阴极获得的H 2 O 2浓度分别高出4.3倍和1.5倍。这种令人惊讶的增强是由于多孔阴极内部三相界面的形成造成的,其中来自环境空气的O 2也被用于H 2 O 2的生产。来自环境空气的O 2的贡献根据阴极材料而变化,并且经计算,GF阴极为76.7%,RVC泡沫阴极为35.6%。评估了 pH、电流和混合对 H 2 O 2产生的影响。最后,通过“浮动”阴极增强的 EF 过程在 120 分钟后降解了 78.3% 的抗炎药物布洛芬,而使用传统的“浸没”电极仅降解了 25.4%,且成本没有增加。










































 京公网安备 11010802027423号
京公网安备 11010802027423号