当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Degradable polymer prodrugs with adjustable activity from drug-initiated radical ring-opening copolymerization†
Chemical Science ( IF 7.6 ) Pub Date : 2018-09-13 00:00:00 , DOI: 10.1039/c8sc02256a Elise Guégain 1 , Johanna Tran 1 , Quentin Deguettes 1 , Julien Nicolas 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-09-13 00:00:00 , DOI: 10.1039/c8sc02256a Elise Guégain 1 , Johanna Tran 1 , Quentin Deguettes 1 , Julien Nicolas 1
Affiliation
|
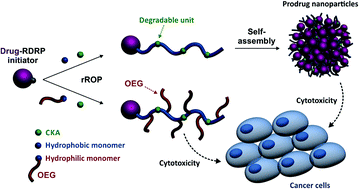
Degradable polymer prodrugs based on gemcitabine (Gem) as an anticancer drug were synthesized by ‘drug-initiated’ nitroxide-mediated radical ring-opening copolymerization (NMrROP) of methacrylic esters and 2-methylene-4-phenyl-1,3-dioxolane (MPDL). Different structural parameters were varied to determine the best biological performances: the nature of the monomer [i.e., oligo(ethylene glycol) methacrylate (OEGMA) or methyl methacrylate (MMA)], the nature of the Gem-polymer linker (i.e., amide or amide and diglycolate) and the MPDL content in the copolymer. Depending on the nature of the methacrylate monomer, two small libraries of water-soluble copolymer prodrugs and nanoparticles were obtained (Mn ∼10 000 g mol−1, Đ = 1.1–1.5), which exhibited tunable hydrolytic degradation under accelerated conditions governed by the MPDL content. Drug-release profiles in human serum and in vitro anticancer activity on different cell lines enabled preliminary structure–activity relationships to be established. The cytotoxicity was independently governed by: (i) the MPDL content – the lower the MPDL content, the greater the cytotoxicity; (ii) the nature of the linker – the presence of a labile diglycolate linker enabled a greater Gem release compared to a simple amide bond and (iii) the hydrophilicity of the methacrylate monomer–OEGMA enabled a greater anticancer activity to be obtained compared to MMA-based polymer prodrugs. Remarkably, the optimal structural parameters enabled reaching the cytotoxic activity of the parent (free) drug.
中文翻译:

通过药物引发的自由基开环共聚可调节活性的可降解聚合物前药†
基于吉西他滨(Gem)作为抗癌药物的可降解聚合物前药是通过甲基丙烯酸酯和 2-亚甲基-4-苯基-1,3-二氧戊环的“药物引发”硝基氧介导的自由基开环共聚(NMrROP)合成的。 MPDL)。改变不同的结构参数以确定最佳的生物性能:单体的性质[即低聚(乙二醇)甲基丙烯酸酯(OEGMA)或甲基丙烯酸甲酯(MMA)]、Gem聚合物连接体的性质(即酰胺或酰胺和二甘醇酸酯)和共聚物中的 MPDL 含量。根据甲基丙烯酸酯单体的性质,获得了两个小的水溶性共聚物前药和纳米颗粒库( M n ∼10 000 g mol -1 , Đ = 1.1–1.5),它们在加速条件下表现出可调节的水解降解MPDL 内容。人血清中的药物释放曲线和不同细胞系的体外抗癌活性使得初步的结构-活性关系得以建立。细胞毒性独立地受以下因素影响:(i) MPDL 含量——MPDL 含量越低,细胞毒性越大; (ii) 连接体的性质——与简单的酰胺键相比,不稳定的二甘醇酸酯连接体的存在能够实现更大的 Gem 释放;(iii) 甲基丙烯酸酯单体的亲水性——OEGMA 与 MMA 相比能够获得更大的抗癌活性基于聚合物的前药。值得注意的是,最佳结构参数能够达到母体(游离)药物的细胞毒活性。
更新日期:2018-09-13
中文翻译:

通过药物引发的自由基开环共聚可调节活性的可降解聚合物前药†
基于吉西他滨(Gem)作为抗癌药物的可降解聚合物前药是通过甲基丙烯酸酯和 2-亚甲基-4-苯基-1,3-二氧戊环的“药物引发”硝基氧介导的自由基开环共聚(NMrROP)合成的。 MPDL)。改变不同的结构参数以确定最佳的生物性能:单体的性质[即低聚(乙二醇)甲基丙烯酸酯(OEGMA)或甲基丙烯酸甲酯(MMA)]、Gem聚合物连接体的性质(即酰胺或酰胺和二甘醇酸酯)和共聚物中的 MPDL 含量。根据甲基丙烯酸酯单体的性质,获得了两个小的水溶性共聚物前药和纳米颗粒库( M n ∼10 000 g mol -1 , Đ = 1.1–1.5),它们在加速条件下表现出可调节的水解降解MPDL 内容。人血清中的药物释放曲线和不同细胞系的体外抗癌活性使得初步的结构-活性关系得以建立。细胞毒性独立地受以下因素影响:(i) MPDL 含量——MPDL 含量越低,细胞毒性越大; (ii) 连接体的性质——与简单的酰胺键相比,不稳定的二甘醇酸酯连接体的存在能够实现更大的 Gem 释放;(iii) 甲基丙烯酸酯单体的亲水性——OEGMA 与 MMA 相比能够获得更大的抗癌活性基于聚合物的前药。值得注意的是,最佳结构参数能够达到母体(游离)药物的细胞毒活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号