Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-09-12 , DOI: 10.1016/j.bioorg.2018.09.004 Urszula Majcher , Alicja Urbaniak , Ewa Maj , Mahshad Moshari , Magdalena Delgado , Joanna Wietrzyk , Franz Bartl , Timothy C. Chambers , Jack A. Tuszynski , Adam Huczyński
|
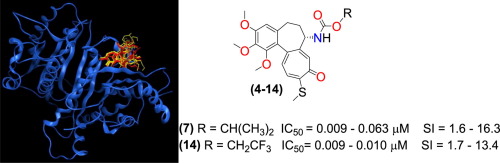
A number of naturally occurring compounds such as paclitaxel, vinblastine, combretastatin, and colchicine exert their therapeutic effect by changing the dynamics of tubulin and its polymer form, microtubules. The identification of tubulin as a potential target for anticancer drugs has led to extensive research followed by clinical development of numerous compounds from several families. In this paper we report on the design, synthesis and in vitro evaluation of a group of thiocolchicine derivatives, modified at ring-B, labelled here compounds 4–14. These compounds have been obtained in a simple reaction of 7-deacetyl-10-thiocolchicine 3 with eleven different alcohols in the presence of triphosgene. These novel agents have been checked for anti-proliferative activity against four human cancer cell lines and their mode of action has been confirmed as colchicine binding site inhibition (CBSI) using molecular docking. Molecular simulations provided rational tubulin binding models for the tested compounds. On the basis of in vitro tests, derivatives 4–8 and 14 demonstrated the highest potency against MCF-7, LoVo and A549 tumor cell lines (IC50 values = 0.009–0.014 μM). They were more potent and characterized by a higher selectivity index than several standard chemotherapeutics including cisplatin and doxorubicin as well as unmodified colchicine. Further, studies revealed that colchicine and its several derivatives arrested MCF-7 cells in mitosis, while its selected derivatives caused microtubule depolymerization.
中文翻译:

硫代秋水仙碱氨基甲酸酯的合成,抗增殖活性和分子对接
许多天然存在的化合物(例如紫杉醇,长春碱,康维他汀和秋水仙碱)通过改变微管蛋白及其聚合物形式(微管)的动力学来发挥其治疗作用。将微管蛋白鉴定为抗癌药物的潜在靶标导致了广泛的研究,随后进行了来自多个家族的许多化合物的临床开发。在本文中,我们报告了一组在环B处修饰的硫代秋水仙碱衍生物的设计,合成和体外评估,该化合物在此处标记为化合物4 – 14。这些化合物是通过7-脱乙酰基-10-硫代秋水仙碱3的简单反应获得的在三光气存在下与十一种不同的醇一起使用。这些新型药物已针对四种人类癌细胞系进行了抗增殖活性检查,其作用方式已通过分子对接证实为秋水仙碱结合位点抑制(CBSI)。分子模拟为测试化合物提供了合理的微管蛋白结合模型。上的基础的体外试验中,衍生物4 - 8和14表现出对MCF-7,的LoVo和A549肿瘤细胞系(IC最高效力50值= 0.009–0.014μM)。与包括顺铂和阿霉素以及未修饰秋水仙碱在内的几种标准化学疗法相比,它们更有效并且具有更高的选择性指数。此外,研究表明秋水仙碱及其几种衍生物使MCF-7细胞停滞在有丝分裂中,而其选择的衍生物引起微管解聚。











































 京公网安备 11010802027423号
京公网安备 11010802027423号