当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reconstitution of Mammalian Enzymatic Deacylation Reactions in Live Bacteria Using Native Acylated Substrates.
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2018-09-18 , DOI: 10.1021/acssynbio.8b00314 Emanuel M Avrahami 1 , Shahar Levi 2 , Eyal Zajfman 1 , Clil Regev 2 , Oshrit Ben-David 2 , Eyal Arbely 1, 2
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2018-09-18 , DOI: 10.1021/acssynbio.8b00314 Emanuel M Avrahami 1 , Shahar Levi 2 , Eyal Zajfman 1 , Clil Regev 2 , Oshrit Ben-David 2 , Eyal Arbely 1, 2
Affiliation
|
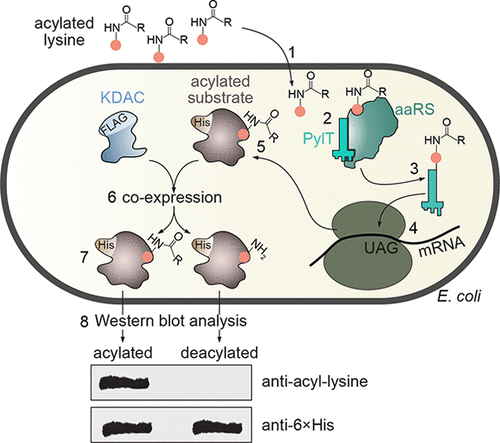
Lysine deacetylases (KDACs) are enzymes that catalyze the hydrolysis of acyl groups from acyl-lysine residues. The recent identification of thousands of putative acylation sites, including specific acetylation sites, created an urgent need for biochemical methodologies aimed at better characterizing KDAC-substrate specificity and evaluating KDACs activity. To address this need, we utilized genetic code expansion technology to coexpress site-specifically acylated substrates with mammalian KDACs, and study substrate recognition and deacylase activity in live Escherichia coli. In this system the bacterial cell serves as a "biological test tube" in which the incubation of a single mammalian KDAC and a potential peptide or full-length acylated substrate transpires. We report novel deacetylation activities of Zn2+-dependent deacetylases and sirtuins in bacteria. We also measure the deacylation of propionyl-, butyryl-, and crotonyl-lysine, as well as novel deacetylation of Lys310-acetylated RelA by SIRT3, SIRT5, SIRT6, and HDAC8. This study highlights the importance of native interactions to KDAC-substrate recognition and deacylase activity.
中文翻译:

使用天然酰化底物重建活细菌中的哺乳动物酶促脱酰反应。
赖氨酸脱乙酰基酶(KDAC)是催化酰基赖氨酸残基水解酰基的酶。最近鉴定了数千个假定的酰化位点,包括特定的乙酰化位点,迫切需要旨在更好地表征KDAC底物特异性和评估KDACs活性的生化方法。为了满足这一需求,我们利用遗传密码扩展技术与哺乳动物KDAC共表达位点特异性酰化的底物,并研究了活大肠杆菌中的底物识别和脱酰基酶活性。在该系统中,细菌细胞充当“生物试管”,单个哺乳动物KDAC和潜在的肽或全长酰化底物的孵育在其中进行。我们报告细菌中依赖于Zn2 +的脱乙酰基酶和sirtuins的新型脱乙酰作用。我们还测量了丙酰,丁酰和巴豆酰赖氨酸的脱酰作用,以及SIRT3,SIRT5,SIRT6和HDAC8对Lys310-乙酰化RelA的新型脱乙酰作用。这项研究强调了天然相互作用对KDAC底物识别和脱酰基酶活性的重要性。
更新日期:2018-09-12
中文翻译:

使用天然酰化底物重建活细菌中的哺乳动物酶促脱酰反应。
赖氨酸脱乙酰基酶(KDAC)是催化酰基赖氨酸残基水解酰基的酶。最近鉴定了数千个假定的酰化位点,包括特定的乙酰化位点,迫切需要旨在更好地表征KDAC底物特异性和评估KDACs活性的生化方法。为了满足这一需求,我们利用遗传密码扩展技术与哺乳动物KDAC共表达位点特异性酰化的底物,并研究了活大肠杆菌中的底物识别和脱酰基酶活性。在该系统中,细菌细胞充当“生物试管”,单个哺乳动物KDAC和潜在的肽或全长酰化底物的孵育在其中进行。我们报告细菌中依赖于Zn2 +的脱乙酰基酶和sirtuins的新型脱乙酰作用。我们还测量了丙酰,丁酰和巴豆酰赖氨酸的脱酰作用,以及SIRT3,SIRT5,SIRT6和HDAC8对Lys310-乙酰化RelA的新型脱乙酰作用。这项研究强调了天然相互作用对KDAC底物识别和脱酰基酶活性的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号