Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cryo-EM structure of the active, Gs-protein complexed, human CGRP receptor
Nature ( IF 50.5 ) Pub Date : 2018-09-01 , DOI: 10.1038/s41586-018-0535-y Yi-Lynn Liang 1 , Maryam Khoshouei 2, 3 , Giuseppe Deganutti 4 , Alisa Glukhova 1 , Cassandra Koole 1 , Thomas S Peat 5 , Mazdak Radjainia 1, 6 , Jürgen M Plitzko 2 , Wolfgang Baumeister 2 , Laurence J Miller 1, 7 , Deborah L Hay 8, 9 , Arthur Christopoulos 1 , Christopher A Reynolds 4 , Denise Wootten 1, 10 , Patrick M Sexton 1, 10
Nature ( IF 50.5 ) Pub Date : 2018-09-01 , DOI: 10.1038/s41586-018-0535-y Yi-Lynn Liang 1 , Maryam Khoshouei 2, 3 , Giuseppe Deganutti 4 , Alisa Glukhova 1 , Cassandra Koole 1 , Thomas S Peat 5 , Mazdak Radjainia 1, 6 , Jürgen M Plitzko 2 , Wolfgang Baumeister 2 , Laurence J Miller 1, 7 , Deborah L Hay 8, 9 , Arthur Christopoulos 1 , Christopher A Reynolds 4 , Denise Wootten 1, 10 , Patrick M Sexton 1, 10
Affiliation
|
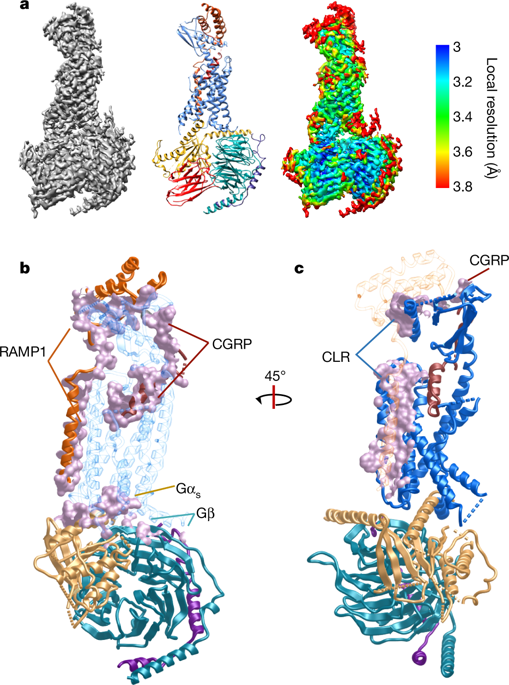
Calcitonin gene-related peptide (CGRP) is a widely expressed neuropeptide that has a major role in sensory neurotransmission. The CGRP receptor is a heterodimer of the calcitonin receptor-like receptor (CLR) class B G-protein-coupled receptor and a type 1 transmembrane domain protein, receptor activity-modifying protein 1 (RAMP1). Here we report the structure of the human CGRP receptor in complex with CGRP and the Gs-protein heterotrimer at 3.3 Å global resolution, determined by Volta phase-plate cryo-electron microscopy. The receptor activity-modifying protein transmembrane domain sits at the interface between transmembrane domains 3, 4 and 5 of CLR, and stabilizes CLR extracellular loop 2. RAMP1 makes only limited direct contact with CGRP, consistent with its function in allosteric modulation of CLR. Molecular dynamics simulations indicate that RAMP1 provides stability to the receptor complex, particularly in the positioning of the extracellular domain of CLR. This work provides insights into the control of G-protein-coupled receptor function.The structure of a complex containing calcitonin gene-related peptide, the human calcitonin gene-related peptide receptor and the Gs heterotrimer, determined using Volta phase-plate cryo-electron microscopy, provides structural insight into the regulation of G-protein-coupled receptors by receptor activity modifying protein 1.
中文翻译:

活性 Gs 蛋白复合的人 CGRP 受体的冷冻电镜结构
降钙素基因相关肽(CGRP)是一种广泛表达的神经肽,在感觉神经传递中发挥重要作用。CGRP 受体是降钙素受体样受体 (CLR) B 类 G 蛋白偶联受体和 1 型跨膜结构域蛋白受体活性修饰蛋白 1 (RAMP1) 的异二聚体。在这里,我们以 3.3 Å 的全局分辨率报告了与 CGRP 和 Gs 蛋白异三聚体复合的人类 CGRP 受体的结构,通过 Volta 相位板冷冻电子显微镜测定。受体活性修饰蛋白跨膜结构域位于 CLR 跨膜结构域 3、4 和 5 之间的界面,并稳定 CLR 细胞外环 2。RAMP1 仅与 CGRP 进行有限的直接接触,这与其在 CLR 变构调节中的功能一致。分子动力学模拟表明 RAMP1 为受体复合物提供稳定性,特别是在 CLR 胞外结构域的定位方面。这项工作为 G 蛋白偶联受体功能的控制提供了见解。使用 Volta 相板低温电子测定了含有降钙素基因相关肽、人降钙素基因相关肽受体和 Gs 异源三聚体的复合物的结构显微镜,提供了受体活性修饰蛋白 1 对 G 蛋白偶联受体调节的结构见解。
更新日期:2018-09-01
中文翻译:

活性 Gs 蛋白复合的人 CGRP 受体的冷冻电镜结构
降钙素基因相关肽(CGRP)是一种广泛表达的神经肽,在感觉神经传递中发挥重要作用。CGRP 受体是降钙素受体样受体 (CLR) B 类 G 蛋白偶联受体和 1 型跨膜结构域蛋白受体活性修饰蛋白 1 (RAMP1) 的异二聚体。在这里,我们以 3.3 Å 的全局分辨率报告了与 CGRP 和 Gs 蛋白异三聚体复合的人类 CGRP 受体的结构,通过 Volta 相位板冷冻电子显微镜测定。受体活性修饰蛋白跨膜结构域位于 CLR 跨膜结构域 3、4 和 5 之间的界面,并稳定 CLR 细胞外环 2。RAMP1 仅与 CGRP 进行有限的直接接触,这与其在 CLR 变构调节中的功能一致。分子动力学模拟表明 RAMP1 为受体复合物提供稳定性,特别是在 CLR 胞外结构域的定位方面。这项工作为 G 蛋白偶联受体功能的控制提供了见解。使用 Volta 相板低温电子测定了含有降钙素基因相关肽、人降钙素基因相关肽受体和 Gs 异源三聚体的复合物的结构显微镜,提供了受体活性修饰蛋白 1 对 G 蛋白偶联受体调节的结构见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号