当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gas Phase Reactivity of the CN Radical with Methyl Amines at Low Temperatures (23–297 K): A Combined Experimental and Theoretical Investigation
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-09-11 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00098 Chantal Sleiman 1 , Gisèle El Dib 1 , Dahbia Talbi 2 , André Canosa 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-09-11 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00098 Chantal Sleiman 1 , Gisèle El Dib 1 , Dahbia Talbi 2 , André Canosa 1
Affiliation
|
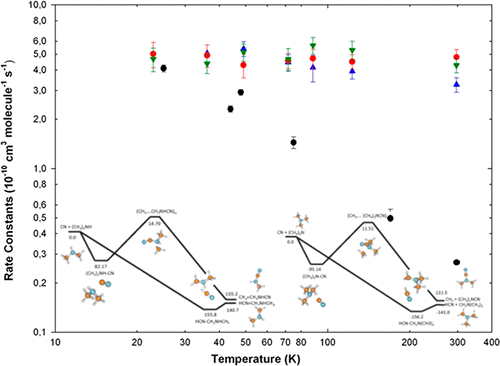
The gas phase reactivity of the CN radical in the presence of dimethylamine (CH3)2NH and trimethylamine (CH3)3N has been investigated experimentally using a uniform supersonic expansion CRESU reactor coupled to the pulsed laser photolysis/laser-induced fluorescence technique. Rate constants have been obtained in the temperature range 23–297 K and were found to be independent of temperature for both reactants. A typical rate constant of 5 × 10–10 cm3 molecule–1 s–1 is representative of the full set of experiments. Additionally, ab initio calculations at the ROCCSD(T)/6-311++G(3df,2pd)//M062X/6-311++G(d,p) level of theory have been carried out in order to determine the energy profiles along the reaction pathways. The paths corresponding to the hydrogen abstraction with the formation of HCN were found to be barrierless and very exothermic for the two studied reactions. Results are compared to previous works concerning the reactivity of CN with ammonia NH3 and methylamine CH3NH2. The energy profile for the reaction with methylamine has been revisited using the level of theory mentioned above. Calculations indicate that hydrogen abstraction is the major reaction pathway as well, in contradiction with a previous investigation claiming that the CN/CH3 substitution is the dominant exit channel.
中文翻译:

低温(23–297 K)下CN自由基与甲基胺的气相反应性:组合的实验和理论研究
使用偶合的超音速膨胀CRESU反应器结合脉冲激光光解/激光诱导荧光技术,对二甲胺(CH 3)2 NH和三甲胺(CH 3)3 N存在下CN自由基的气相反应性进行了实验研究。 。在23–297 K的温度范围内已获得速率常数,并且发现这两种反应物的速率常数均与温度无关。5×10 –10 cm 3分子的典型速率常数–1 s –1是整套实验的代表。此外,已经在ROCCSD(T)/ 6-311 ++ G(3df,2pd)// M062X / 6-311 ++ G(d,p)的理论水平上进行了从头计算,以确定沿反应路径的能量分布。发现与形成HCN的氢提取相对应的路径对于两个研究的反应而言是无障碍的并且非常放热。将结果与先前有关CN与氨NH 3和甲胺CH 3 NH 2的反应性的工作进行比较。与甲胺反应的能量分布已经使用上述理论水平进行了重新讨论。计算表明,提取氢气也是主要的反应途径,这与先前的声称CN / CH 3取代是主要的出口通道的研究相矛盾。
更新日期:2018-09-11
中文翻译:

低温(23–297 K)下CN自由基与甲基胺的气相反应性:组合的实验和理论研究
使用偶合的超音速膨胀CRESU反应器结合脉冲激光光解/激光诱导荧光技术,对二甲胺(CH 3)2 NH和三甲胺(CH 3)3 N存在下CN自由基的气相反应性进行了实验研究。 。在23–297 K的温度范围内已获得速率常数,并且发现这两种反应物的速率常数均与温度无关。5×10 –10 cm 3分子的典型速率常数–1 s –1是整套实验的代表。此外,已经在ROCCSD(T)/ 6-311 ++ G(3df,2pd)// M062X / 6-311 ++ G(d,p)的理论水平上进行了从头计算,以确定沿反应路径的能量分布。发现与形成HCN的氢提取相对应的路径对于两个研究的反应而言是无障碍的并且非常放热。将结果与先前有关CN与氨NH 3和甲胺CH 3 NH 2的反应性的工作进行比较。与甲胺反应的能量分布已经使用上述理论水平进行了重新讨论。计算表明,提取氢气也是主要的反应途径,这与先前的声称CN / CH 3取代是主要的出口通道的研究相矛盾。











































 京公网安备 11010802027423号
京公网安备 11010802027423号