当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal‐free Deoxygenative 2‐Amidation of Quinoline N‐oxides with Nitriles via a Radical Activation Pathway
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-10-08 , DOI: 10.1002/adsc.201800918 Long‐Yong Xie 1 , Sha Peng 1 , Fang Liu 1 , Jin‐Yu Yi 1 , Ming Wang 2 , Zilong Tang 2 , Xinhua Xu 3 , Wei‐Min He 1, 3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2018-10-08 , DOI: 10.1002/adsc.201800918 Long‐Yong Xie 1 , Sha Peng 1 , Fang Liu 1 , Jin‐Yu Yi 1 , Ming Wang 2 , Zilong Tang 2 , Xinhua Xu 3 , Wei‐Min He 1, 3
Affiliation
|
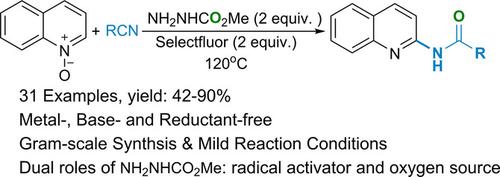
A metal‐, base‐ and reductant‐free approach for the efficient synthesis of various N‐acylated 2‐aminoquinolines was reported. In this work, readily available nitriles are used as the amide source, and methyl carbazate as both the radical activating reagent and oxygen source. This is the first report on the ester‐radical‐activated highly regioselective addition of nitriles to quinolone N‐oxides. This procedure is expected to complement the current methods for functionalization of N‐oxides via an electrophilic activation mechanism.
中文翻译:

通过自由基活化途径将金属喹啉N-氧化物与腈进行无金属脱氧2-酰胺化反应
报道了一种无金属,无碱和无还原剂的方法,可有效合成各种N酰化的2-氨基喹啉。在这项工作中,将现成的腈用作酰胺源,将氨基甲酸甲酯用作自由基活化剂和氧源。这是第一个关于酯基活化的高区域选择性将腈加到喹诺酮N-氧化物上的报道。预计该程序将补充当前通过亲电子激活机制对N氧化物进行功能化的方法。
更新日期:2018-10-08
中文翻译:

通过自由基活化途径将金属喹啉N-氧化物与腈进行无金属脱氧2-酰胺化反应
报道了一种无金属,无碱和无还原剂的方法,可有效合成各种N酰化的2-氨基喹啉。在这项工作中,将现成的腈用作酰胺源,将氨基甲酸甲酯用作自由基活化剂和氧源。这是第一个关于酯基活化的高区域选择性将腈加到喹诺酮N-氧化物上的报道。预计该程序将补充当前通过亲电子激活机制对N氧化物进行功能化的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号