当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Divergent Reactivity of Stannane and Silane in the Trifluoromethylation of PdII: Cyclic Transition State versus Difluorocarbene Release
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-10-17 , DOI: 10.1002/anie.201808229 Maoping Pu 1 , Italo A. Sanhueza 1, 2 , Erdem Senol 1 , Franziska Schoenebeck 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-10-17 , DOI: 10.1002/anie.201808229 Maoping Pu 1 , Italo A. Sanhueza 1, 2 , Erdem Senol 1 , Franziska Schoenebeck 1
Affiliation
|
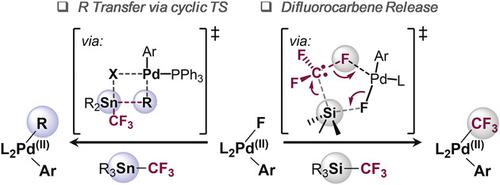
The transmetalation is a key elementary step in cross‐coupling reactions. Yet, the precise nature of its mechanism and transition state geometry are frequently elusive. This report discloses our study of the transmetalation of [PdII]‐F complexes with the silane‐ and stannane‐based trifluoromethylation agents, R3SiCF3 and R3SnCF3. A divergent reactivity was uncovered, with the stannane showing selective R‐group transfer, and the silane selective CF3‐group transfer. Using a combined experimental and computational approach, we uncovered a hitherto unrecognized transmetalation mechanism with the widely employed R3SiCF3 reagent, explaining its unique activity in metal‐catalyzed trifluoromethylations. While the stannane reacts via a cyclic, 4‐membered transition state, the silane undergoes a fundamentally different pathway and releases a difluorocarbene in the transmetalation event. Molecular dynamics studies clearly reinforced the liberation of a free CF2 carbene, which reacts with [PdII]‐F to ultimately generate [PdII]‐CF3.
中文翻译:

PdII的三氟甲基化反应中锡烷和硅烷的不同反应性:循环过渡态与二氟卡宾的释放
重金属化是交叉偶联反应中的关键基本步骤。然而,其机理和过渡态几何形状的精确性质常常难以捉摸。该报告公开了我们对[Pd II ] -F配合物与硅烷和锡烷基三氟甲基化剂R 3 SiCF 3和R 3 SnCF 3的金属转移的研究。发现了不同的反应性,其中锡烷显示出选择性的R-基团转移,而硅烷显示了选择性的CF 3-基团转移。使用组合的实验和计算方法,我们发现了R 3 SiCF 3被广泛采用的迄今无法识别的重金属化机理试剂,解释了其在金属催化的三氟甲基化反应中的独特活性。锡烷通过环状的四元过渡态反应时,硅烷经历了根本不同的途径,并在重金属化事件中释放出二氟卡宾。分子动力学研究清楚地增强了游离CF 2卡宾的释放,其与[Pd II ] -F反应最终生成[Pd II ] -CF 3。
更新日期:2018-10-17
中文翻译:

PdII的三氟甲基化反应中锡烷和硅烷的不同反应性:循环过渡态与二氟卡宾的释放
重金属化是交叉偶联反应中的关键基本步骤。然而,其机理和过渡态几何形状的精确性质常常难以捉摸。该报告公开了我们对[Pd II ] -F配合物与硅烷和锡烷基三氟甲基化剂R 3 SiCF 3和R 3 SnCF 3的金属转移的研究。发现了不同的反应性,其中锡烷显示出选择性的R-基团转移,而硅烷显示了选择性的CF 3-基团转移。使用组合的实验和计算方法,我们发现了R 3 SiCF 3被广泛采用的迄今无法识别的重金属化机理试剂,解释了其在金属催化的三氟甲基化反应中的独特活性。锡烷通过环状的四元过渡态反应时,硅烷经历了根本不同的途径,并在重金属化事件中释放出二氟卡宾。分子动力学研究清楚地增强了游离CF 2卡宾的释放,其与[Pd II ] -F反应最终生成[Pd II ] -CF 3。











































 京公网安备 11010802027423号
京公网安备 11010802027423号