当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Overview of Methane Hydroxylation by Copper–Oxygen Species in Enzymatic and Zeolitic Catalysts
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-09-12 00:00:00 , DOI: 10.1021/acs.accounts.8b00236 M. Haris Mahyuddin 1 , Yoshihito Shiota 1 , Aleksandar Staykov 2 , Kazunari Yoshizawa 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-09-12 00:00:00 , DOI: 10.1021/acs.accounts.8b00236 M. Haris Mahyuddin 1 , Yoshihito Shiota 1 , Aleksandar Staykov 2 , Kazunari Yoshizawa 1
Affiliation
|
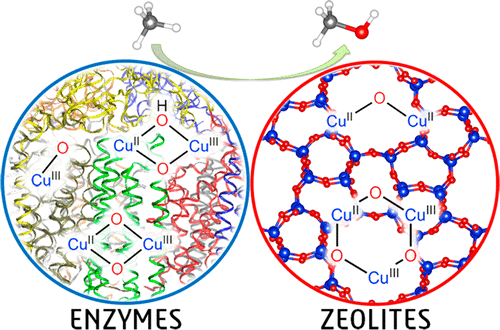
As fossil-based energy sources become more depleted and with renewable-energy technologies still in a very early stage of development, the utilization of highly abundant methane as a transitional solution for current energy demands is highly important despite difficulties in transport and storage. Technologies enabling the conversion of methane to liquid/condensable energy carriers that can be easily transported and integrated into the existing chemical infrastructures are therefore essential. Although there commercially exists a two-step gas-to-liquid process involving syngas production, a novel route of methane conversion that can circumvent the high-cost production of syngas should be developed. Among all of the conceptually possible methods for converting methane to methanol, methane hydroxylation (CH4 + 1/2O2 → CH3OH) at low temperature seems to be the most viable since it provides a direct route of conversion and allows a much lower operational cost. However, it is hampered by the fact that the complete oxidation to CO2 is thermodynamically more favored. To overcome this, an effective catalyst that is able to “mildly” oxidize methane and stabilize the resultant methyl radical toward methanol formation is required. Particulate methane monooxygenase (pMMO) and copper-exchanged zeolites are two catalysts known to hydroxylate methane into methanol at low temperature with high selectivity. Having been studied for more than 30 years, these copper-cored catalysts are still relevant topics of discussion since the actual structure of the active sites has not been agreed upon, and thus, the reaction mechanism and factors influencing their reactivity and productivity are yet to be understood. Density functional theory (DFT) has provided us with a powerful computational tool for accomplishing these tasks.
中文翻译:

酶和沸石催化剂中铜氧物种甲烷羟化反应的理论概述
随着基于化石的能源越来越枯竭,可再生能源技术仍处于发展的早期阶段,尽管运输和存储存在困难,但利用高度丰富的甲烷作为当前能源需求的过渡解决方案仍然非常重要。因此,必不可少的技术是将甲烷转化为可轻松运输并整合到现有化学基础设施中的液态/可冷凝能源载体。尽管商业上存在涉及合成气生产的两步法气-液工艺,但是应该开发一种可以避免合成气高成本生产的甲烷转化新路线。在将甲烷转化为甲醇的所有概念上可能的方法中,甲烷羟基化(CH 4 +1 / 2 ö 2 →CH 3在低温下OH)似乎是最可行的,因为它提供的转换的直接路由,并且允许低得多的运作成本。然而,它被以下事实所阻碍:完全氧化为CO 2在热力学上更受青睐。为了克服这个问题,需要一种能够“温和”地氧化甲烷并使形成的甲基自由基趋向于形成甲醇的有效催化剂。颗粒状的甲烷单加氧酶(pMMO)和铜交换的沸石是已知的在低温下以高选择性将甲烷羟化为甲醇的两种催化剂。经过30多年的研究,这些铜芯催化剂仍然是讨论的主题,因为尚未就活性位点的实际结构达成一致,因此反应机理和影响其反应性和生产率的因素尚待探讨。被理解。密度泛函理论(DFT)为我们提供了完成这些任务的强大计算工具。
更新日期:2018-09-12
中文翻译:

酶和沸石催化剂中铜氧物种甲烷羟化反应的理论概述
随着基于化石的能源越来越枯竭,可再生能源技术仍处于发展的早期阶段,尽管运输和存储存在困难,但利用高度丰富的甲烷作为当前能源需求的过渡解决方案仍然非常重要。因此,必不可少的技术是将甲烷转化为可轻松运输并整合到现有化学基础设施中的液态/可冷凝能源载体。尽管商业上存在涉及合成气生产的两步法气-液工艺,但是应该开发一种可以避免合成气高成本生产的甲烷转化新路线。在将甲烷转化为甲醇的所有概念上可能的方法中,甲烷羟基化(CH 4 +1 / 2 ö 2 →CH 3在低温下OH)似乎是最可行的,因为它提供的转换的直接路由,并且允许低得多的运作成本。然而,它被以下事实所阻碍:完全氧化为CO 2在热力学上更受青睐。为了克服这个问题,需要一种能够“温和”地氧化甲烷并使形成的甲基自由基趋向于形成甲醇的有效催化剂。颗粒状的甲烷单加氧酶(pMMO)和铜交换的沸石是已知的在低温下以高选择性将甲烷羟化为甲醇的两种催化剂。经过30多年的研究,这些铜芯催化剂仍然是讨论的主题,因为尚未就活性位点的实际结构达成一致,因此反应机理和影响其反应性和生产率的因素尚待探讨。被理解。密度泛函理论(DFT)为我们提供了完成这些任务的强大计算工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号