当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Basis for Autocatalytic Backbone N-Methylation in RiPP Natural Product Biosynthesis
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-09-11 00:00:00 , DOI: 10.1021/acschembio.8b00668 Chayanid Ongpipattanakul , Satish K. Nair
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2018-09-11 00:00:00 , DOI: 10.1021/acschembio.8b00668 Chayanid Ongpipattanakul , Satish K. Nair
|
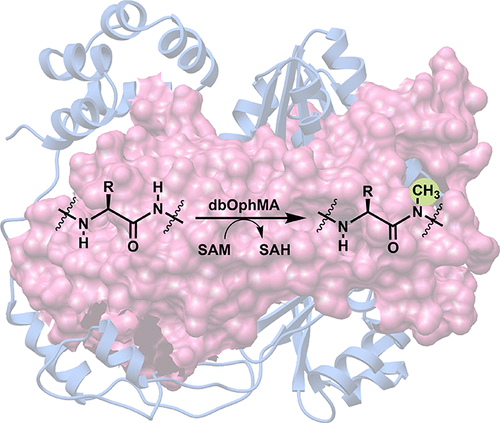
N-methylation of nucleic acids, proteins, and peptides is a chemical modification with significant impact on biological regulation. Despite the simplicity of the structural change, N-methylation can influence diverse functions including epigenetics, protein complex formation, and microtubule stability. While there are limited examples of N-methylation of the α-amino group of bacterial and eukaryotic proteins, there are no examples of catalysts that carry out post-translation methylation of backbone amides in proteins or peptides. Recent studies have identified enzymes that catalyze backbone N-methylation on a peptide substrate, a reaction with little biochemical precedent, in a family of ribosomally synthesized natural products produced in basidiomycetes. Here, we describe the crystal structures of Dendrothele bispora dbOphMA, a methyltransferase that catalyzes multiple N-methylations on the peptide backbone. We further carry out biochemical studies of this catalyst to determine the molecular details that promote this unusual chemical transformation. The structural and biochemical framework described here could facilitate biotechnological applications of catalysts for the rapid production of backbone N-methylated peptides.
中文翻译:

RiPP天然产物生物合成中自催化主链N-甲基化的分子基础
核酸,蛋白质和肽的N-甲基化是一种化学修饰,会对生物调节产生重大影响。尽管结构改变很简单,但是N-甲基化可以影响多种功能,包括表观遗传学,蛋白质复合物形成和微管稳定性。尽管细菌和真核蛋白质的α-氨基的N-甲基化的例子有限,但是没有进行蛋白质或肽中主链酰胺的翻译后甲基化的催化剂的例子。最近的研究已经鉴定出催化主链N的酶-在担子菌中产生的核糖体合成天然产物家族中,在肽底物上进行甲基化反应,这几乎没有生化先例。在这里,我们描述了Dendrothele bispora dbOphMA的晶体结构,这是一种甲基转移酶,可催化肽主链上的多个N-甲基化。我们进一步对该催化剂进行生化研究,以确定促进这种异常化学转化的分子细节。本文所述的结构和生化框架可以促进催化剂的生物技术应用,以快速生产骨架N-甲基化肽。
更新日期:2018-09-11
中文翻译:

RiPP天然产物生物合成中自催化主链N-甲基化的分子基础
核酸,蛋白质和肽的N-甲基化是一种化学修饰,会对生物调节产生重大影响。尽管结构改变很简单,但是N-甲基化可以影响多种功能,包括表观遗传学,蛋白质复合物形成和微管稳定性。尽管细菌和真核蛋白质的α-氨基的N-甲基化的例子有限,但是没有进行蛋白质或肽中主链酰胺的翻译后甲基化的催化剂的例子。最近的研究已经鉴定出催化主链N的酶-在担子菌中产生的核糖体合成天然产物家族中,在肽底物上进行甲基化反应,这几乎没有生化先例。在这里,我们描述了Dendrothele bispora dbOphMA的晶体结构,这是一种甲基转移酶,可催化肽主链上的多个N-甲基化。我们进一步对该催化剂进行生化研究,以确定促进这种异常化学转化的分子细节。本文所述的结构和生化框架可以促进催化剂的生物技术应用,以快速生产骨架N-甲基化肽。











































 京公网安备 11010802027423号
京公网安备 11010802027423号