Journal of Solid State Chemistry ( IF 3.2 ) Pub Date : 2018-09-12 , DOI: 10.1016/j.jssc.2018.09.015 Isabel Iglesias , Belén F. Alfonso , Camino Trobajo , José A. Huidobro , Zakariae Amghouz , David Martínez-Blanco , Jesús A. Blanco , José R. García
|
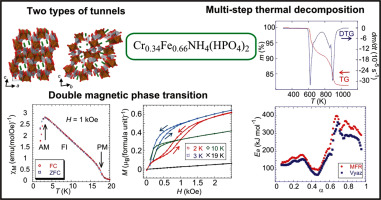
The hydrothermal synthesis and the chemical-physical characterization of an ammonium-chromium(III)-iron(III) bis(hydrogenphosphate), Cr0.34Fe0.66NH4(HPO4)2 [CrFeNP] is reported. It was obtained in aqueous media by reaction between chromium(III) chloride, iron(III) chloride, urea, and orthophosphoric acid. The crystal structure of CrFeNP was determined from single-crystal X-ray diffraction data. It crystallizes in the triclinic system, space group P−1, and exhibits two types of different infinite tunnels along [0 1 0] and [1 0 0] directions, where the ammonium cations are located. Thermal analysis shows that the solid is stable up to ca. 600 K. The activation energy of the thermal decomposition up to 1273 K was computed by isoconversional methods. In addition, the magnetic behaviour of the material was investigated from magnetic susceptibility and magnetization measurements. CrFeNP undergoes two successive magnetic transitions at temperatures K and K, which could be related to a ferri- and an antiferromagnetic magnetic phase transitions, respectively.
中文翻译:

新型铵-铬-铁(III)双(磷酸氢盐)的水热合成,晶体结构,热行为和磁性能
报道了铬-铬(III)-铁(III)双(磷酸氢根)Cr 0.34 Fe 0.66 NH 4(HPO 4)2 [CrFeNP]的水热合成和化学物理性质。在水性介质中,是通过氯化铬(III),氯化铁(III),尿素和正磷酸之间的反应获得的。CrFeNP的晶体结构由单晶X射线衍射数据确定。它在三斜晶系空间群P -1中结晶,并沿铵离子位于[0 1 0]和[1 0 0]的方向呈现出两种类型的无限隧道。热分析表明,该固体稳定至约200℃。600 K. 通过等转换方法计算了高达1273 K的热分解活化能。此外,还从磁化率和磁化强度测量中研究了材料的磁性能。CrFeNP在温度下经历了两个连续的磁跃迁 K和 K,可能分别与亚铁磁性和反铁磁性磁性相变有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号