Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Millifluidic culture improves human midbrain organoid vitality and differentiation†
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-09-11 00:00:00 , DOI: 10.1039/c8lc00206a Emanuel Berger 1, 2, 3 , Chiara Magliaro 4, 5, 6 , Nicole Paczia 1, 3, 7 , Anna S. Monzel 1, 2, 3 , Paul Antony 1, 3, 8 , Carole L. Linster 1, 3, 7 , Silvia Bolognin 1, 2, 3 , Arti Ahluwalia 4, 5, 6 , Jens C. Schwamborn 1, 2, 3
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-09-11 00:00:00 , DOI: 10.1039/c8lc00206a Emanuel Berger 1, 2, 3 , Chiara Magliaro 4, 5, 6 , Nicole Paczia 1, 3, 7 , Anna S. Monzel 1, 2, 3 , Paul Antony 1, 3, 8 , Carole L. Linster 1, 3, 7 , Silvia Bolognin 1, 2, 3 , Arti Ahluwalia 4, 5, 6 , Jens C. Schwamborn 1, 2, 3
Affiliation
|
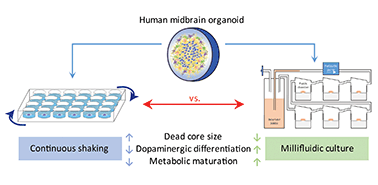
Human midbrain-specific organoids (hMOs) serve as an experimental in vitro model for studying the pathogenesis of Parkinson's disease (PD). In hMOs, neuroepithelial stem cells (NESCs) give rise to functional midbrain dopaminergic (mDA) neurons that are selectively degenerating during PD. A limitation of the hMO model is an under-supply of oxygen and nutrients to the densely packed core region, which leads eventually to a “dead core”. To reduce this phenomenon, we applied a millifluidic culture system that ensures media supply by continuous laminar flow. We developed a computational model of oxygen transport and consumption in order to predict oxygen levels within the hMOs. The modelling predicts higher oxygen levels in the hMO core region under millifluidic conditions. In agreement with the computational model, a significantly smaller “dead core” was observed in hMOs cultured in a bioreactor system compared to those ones kept under conventional shaking conditions. Comparing the necrotic core regions in the organoids with those obtained from the model allowed an estimation of the critical oxygen concentration necessary for ensuring cell vitality. Besides the reduced “dead core” size, the differentiation efficiency from NESCs to mDA neurons was elevated in hMOs exposed to medium flow. Increased differentiation involved a metabolic maturation process that was further developed in the millifluidic culture. Overall, bioreactor conditions that improve hMO quality are worth considering in the context of advanced PD modelling.
中文翻译:

微流培养提高了人类中脑类器官的活力和分化能力†
人类中脑特异性类器官(hMOs)用作体外实验用于研究帕金森氏病(PD)发病机理的模型。在hMOs中,神经上皮干细胞(NESCs)产生功能性中脑多巴胺能(mDA)神经元,该神经元在PD期间选择性退化。hMO模型的局限性在于核心区域密密麻麻的氧气和养分供应不足,最终导致“核心枯竭”。为了减少这种现象,我们应用了微流控培养系统,以确保通过连续层流提供培养基。我们开发了氧气运输和消耗的计算模型,以便预测hMO中的氧气水平。该模型预测在微流体条件下,hMO核心区域的氧含量更高。与计算模型一致,与在常规振荡条件下保存的生物相比,在生物反应器系统中培养的hMO中观察到的“死芯”要小得多。将类器官中的坏死核心区域与从模型中获得的核心区域进行比较,可以估算确保细胞活力所必需的临界氧浓度。除了减小“死芯”大小外,在暴露于中等流量的hMO中,从NESCs向mDA神经元的分化效率也得到了提高。增加的分化涉及代谢成熟过程,该过程在微流控培养中进一步发展。总体而言,在高级PD建模的背景下,值得考虑的是提高hMO质量的生物反应器条件。将类器官中的坏死核心区域与从模型中获得的核心区域进行比较,可以估算确保细胞活力所必需的临界氧浓度。除了减小“死芯”大小外,在暴露于中等流量的hMO中,从NESCs向mDA神经元的分化效率也得到了提高。增加的分化涉及代谢成熟过程,该过程在微流控培养中进一步发展。总体而言,在高级PD建模的背景下,值得考虑的是提高hMO质量的生物反应器条件。将类器官中的坏死核心区域与从模型中获得的核心区域进行比较,可以估算确保细胞活力所必需的临界氧浓度。除了减小“死芯”大小外,在暴露于中等流量的hMO中,从NESCs向mDA神经元的分化效率也得到了提高。增加的分化涉及代谢成熟过程,该过程在微流控培养中进一步发展。总体而言,在高级PD建模的背景下,值得考虑的是提高hMO质量的生物反应器条件。增加的分化涉及代谢成熟过程,该过程在微流控培养中进一步发展。总体而言,在高级PD建模的背景下,值得考虑的是提高hMO质量的生物反应器条件。增加的分化涉及代谢成熟过程,该过程在微流控培养中进一步发展。总体而言,在高级PD建模的背景下,值得考虑的是提高hMO质量的生物反应器条件。
更新日期:2018-09-11
中文翻译:

微流培养提高了人类中脑类器官的活力和分化能力†
人类中脑特异性类器官(hMOs)用作体外实验用于研究帕金森氏病(PD)发病机理的模型。在hMOs中,神经上皮干细胞(NESCs)产生功能性中脑多巴胺能(mDA)神经元,该神经元在PD期间选择性退化。hMO模型的局限性在于核心区域密密麻麻的氧气和养分供应不足,最终导致“核心枯竭”。为了减少这种现象,我们应用了微流控培养系统,以确保通过连续层流提供培养基。我们开发了氧气运输和消耗的计算模型,以便预测hMO中的氧气水平。该模型预测在微流体条件下,hMO核心区域的氧含量更高。与计算模型一致,与在常规振荡条件下保存的生物相比,在生物反应器系统中培养的hMO中观察到的“死芯”要小得多。将类器官中的坏死核心区域与从模型中获得的核心区域进行比较,可以估算确保细胞活力所必需的临界氧浓度。除了减小“死芯”大小外,在暴露于中等流量的hMO中,从NESCs向mDA神经元的分化效率也得到了提高。增加的分化涉及代谢成熟过程,该过程在微流控培养中进一步发展。总体而言,在高级PD建模的背景下,值得考虑的是提高hMO质量的生物反应器条件。将类器官中的坏死核心区域与从模型中获得的核心区域进行比较,可以估算确保细胞活力所必需的临界氧浓度。除了减小“死芯”大小外,在暴露于中等流量的hMO中,从NESCs向mDA神经元的分化效率也得到了提高。增加的分化涉及代谢成熟过程,该过程在微流控培养中进一步发展。总体而言,在高级PD建模的背景下,值得考虑的是提高hMO质量的生物反应器条件。将类器官中的坏死核心区域与从模型中获得的核心区域进行比较,可以估算确保细胞活力所必需的临界氧浓度。除了减小“死芯”大小外,在暴露于中等流量的hMO中,从NESCs向mDA神经元的分化效率也得到了提高。增加的分化涉及代谢成熟过程,该过程在微流控培养中进一步发展。总体而言,在高级PD建模的背景下,值得考虑的是提高hMO质量的生物反应器条件。增加的分化涉及代谢成熟过程,该过程在微流控培养中进一步发展。总体而言,在高级PD建模的背景下,值得考虑的是提高hMO质量的生物反应器条件。增加的分化涉及代谢成熟过程,该过程在微流控培养中进一步发展。总体而言,在高级PD建模的背景下,值得考虑的是提高hMO质量的生物反应器条件。











































 京公网安备 11010802027423号
京公网安备 11010802027423号