当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tuning the Electron Affinity and Stacking Properties of Corannulene by Introduction of Fluorinated Thioethers
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-09-24 , DOI: 10.1002/asia.201801311 Axel Haupt 1 , Dieter Lentz 1
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-09-24 , DOI: 10.1002/asia.201801311 Axel Haupt 1 , Dieter Lentz 1
Affiliation
|
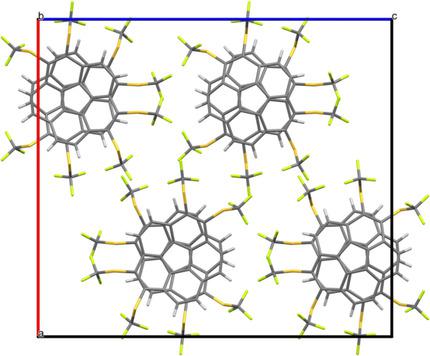
Trifluoromethylthio‐substituted corannulene can be easily synthesized in good yield by the reaction of iodocorannulene with CuSCF3. Oxidation with meta‐chloroperbenzoic acid (mCPBA) yields the corresponding sulfonyl compound which exhibits the largest anodic shift of the redox potential caused by a single substituent. Similarly, four SCF3, SC6F5 and SeC6F5 substituents are introduced in the 1,2,5,6‐positions of corannulene starting with 1,2,5,6‐tetraiodo‐ or 1,2,5,6‐tetrabromocorannulene, respectively. The reactions are performed in polar aprotic solvents and are believed to follow SNAr‐type substitution mechanisms. Crystal and molecular structures of selected compounds were elucidated by X‐ray crystallography. Trifluoromethanesulfonyl corannulene and the fourfold‐substituted trifluoromethylthioether exhibit a perfect columnar stacking of the bowls. The substituted corannulenes were investigated electrochemically by cyclic voltammetry giving raise to large anodic shifts owing to substitution with electron‐withdrawing groups.
中文翻译:

通过引入氟化硫醚来调节邻苯二酚的电子亲和力和堆积特性
通过碘二十二碳六烯与CuSCF 3的反应,可以轻松以高收率轻松合成三氟甲硫基取代的氢化萘。氧化用的元氯过苯甲酸(米CPBA)得到其表现出引起的单一取代基的氧化还原电位的最大阳极移相应的磺酰基化合物。同样地,在1,2,5,6-四碘或1,2,5、1,2,5,6,5,6-三氢化萘中的1,2,5,6-位引入了四个SCF 3,SC 6 F 5和SeC 6 F 5取代基,分别为6-四溴邻并戊二烯。该反应在极性非质子溶剂中进行,据信遵循S NAr型替代机制。通过X射线晶体学阐明了所选化合物的晶体和分子结构。三氟甲磺酰基cor喃烯和四倍取代的三氟甲硫醚表现出碗的完美柱状堆积。通过循环伏安法对取代的Corannulenes进行了电化学研究,由于吸电子基团的取代,产生了较大的阳极位移。
更新日期:2018-09-24
中文翻译:

通过引入氟化硫醚来调节邻苯二酚的电子亲和力和堆积特性
通过碘二十二碳六烯与CuSCF 3的反应,可以轻松以高收率轻松合成三氟甲硫基取代的氢化萘。氧化用的元氯过苯甲酸(米CPBA)得到其表现出引起的单一取代基的氧化还原电位的最大阳极移相应的磺酰基化合物。同样地,在1,2,5,6-四碘或1,2,5、1,2,5,6,5,6-三氢化萘中的1,2,5,6-位引入了四个SCF 3,SC 6 F 5和SeC 6 F 5取代基,分别为6-四溴邻并戊二烯。该反应在极性非质子溶剂中进行,据信遵循S NAr型替代机制。通过X射线晶体学阐明了所选化合物的晶体和分子结构。三氟甲磺酰基cor喃烯和四倍取代的三氟甲硫醚表现出碗的完美柱状堆积。通过循环伏安法对取代的Corannulenes进行了电化学研究,由于吸电子基团的取代,产生了较大的阳极位移。











































 京公网安备 11010802027423号
京公网安备 11010802027423号