当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovering Selective Binders for Photoswitchable Proteins Using Phage Display
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2018-09-11 00:00:00 , DOI: 10.1021/acssynbio.8b00123 Jakeb M. Reis 1 , Xiuling Xu 1 , Sherin McDonald 2 , Ryan M. Woloschuk 1 , Anna S. I. Jaikaran 1 , Frederick S. Vizeacoumar 2 , G. Andrew Woolley 1 , Maruti Uppalapati 2
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2018-09-11 00:00:00 , DOI: 10.1021/acssynbio.8b00123 Jakeb M. Reis 1 , Xiuling Xu 1 , Sherin McDonald 2 , Ryan M. Woloschuk 1 , Anna S. I. Jaikaran 1 , Frederick S. Vizeacoumar 2 , G. Andrew Woolley 1 , Maruti Uppalapati 2
Affiliation
|
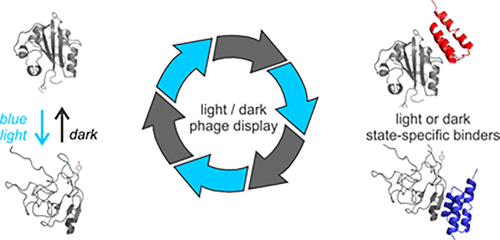
Nature provides an array of proteins that change conformation in response to light. The discovery of a complementary array of proteins that bind only the light-state or dark-state conformation of their photoactive partner proteins would allow each light-switchable protein to be used as an optogenetic tool to control protein–protein interactions. However, as many photoactive proteins have no known binding partner, the advantages of optogenetic control—precise spatial and temporal resolution—are currently restricted to a few well-defined natural systems. In addition, the affinities and kinetics of native interactions are often suboptimal and are difficult to engineer in the absence of any structural information. We report a phage display strategy using a small scaffold protein that can be used to discover new binding partners for both light and dark states of a given light-switchable protein. We used our approach to generate binding partners that interact specifically with the light state or the dark state conformation of two light-switchable proteins: PYP, a test case for a protein with no known partners, and AsLOV2, a well-characterized protein. We show that these novel light-switchable protein–protein interactions can function in living cells to control subcellular localization processes.
中文翻译:

使用噬菌体展示发现光开关蛋白的选择性结合剂
大自然提供了一系列蛋白质,这些蛋白质会响应光而改变构象。发现仅与它们的光敏伴侣蛋白的亮态或暗态构象结合的互补蛋白阵列的发现,将使每种光可转换蛋白都可以用作控制蛋白与蛋白相互作用的光遗传学工具。但是,由于许多光敏蛋白没有已知的结合配偶体,因此光遗传学控制的优势(精确的时空分辨率)目前仅限于一些定义明确的自然系统。另外,天然相互作用的亲和力和动力学常常是次优的,并且在缺乏任何结构信息的情况下很难进行工程设计。我们报告了使用小型支架蛋白的噬菌体展示策略,该蛋白可用于发现给定光开关蛋白的亮态和暗态的新结合伴侣。我们使用我们的方法来生成与两个光可切换蛋白的亮态或暗态构象特异性相互作用的结合伴侣:PYP(一种没有已知伴侣的蛋白质测试用例)和AsLOV2(一种表征良好的蛋白质)。我们证明了这些新颖的光可转换蛋白之间的相互作用可以在活细胞中发挥功能来控制亚细胞定位过程。和AsLOV2(一种特征明确的蛋白质)。我们证明了这些新颖的光可切换的蛋白质-蛋白质相互作用可以在活细胞中起作用,以控制亚细胞定位过程。和AsLOV2(一种特征明确的蛋白质)。我们证明了这些新颖的光可转换蛋白之间的相互作用可以在活细胞中发挥功能来控制亚细胞定位过程。
更新日期:2018-09-11
中文翻译:

使用噬菌体展示发现光开关蛋白的选择性结合剂
大自然提供了一系列蛋白质,这些蛋白质会响应光而改变构象。发现仅与它们的光敏伴侣蛋白的亮态或暗态构象结合的互补蛋白阵列的发现,将使每种光可转换蛋白都可以用作控制蛋白与蛋白相互作用的光遗传学工具。但是,由于许多光敏蛋白没有已知的结合配偶体,因此光遗传学控制的优势(精确的时空分辨率)目前仅限于一些定义明确的自然系统。另外,天然相互作用的亲和力和动力学常常是次优的,并且在缺乏任何结构信息的情况下很难进行工程设计。我们报告了使用小型支架蛋白的噬菌体展示策略,该蛋白可用于发现给定光开关蛋白的亮态和暗态的新结合伴侣。我们使用我们的方法来生成与两个光可切换蛋白的亮态或暗态构象特异性相互作用的结合伴侣:PYP(一种没有已知伴侣的蛋白质测试用例)和AsLOV2(一种表征良好的蛋白质)。我们证明了这些新颖的光可转换蛋白之间的相互作用可以在活细胞中发挥功能来控制亚细胞定位过程。和AsLOV2(一种特征明确的蛋白质)。我们证明了这些新颖的光可切换的蛋白质-蛋白质相互作用可以在活细胞中起作用,以控制亚细胞定位过程。和AsLOV2(一种特征明确的蛋白质)。我们证明了这些新颖的光可转换蛋白之间的相互作用可以在活细胞中发挥功能来控制亚细胞定位过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号