当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unusual Pericyclic Reactivity of 4‐Nitrobenzofuroxans in 1,3‐Dipolar Cycloaddition with N‐Benzyl Azomethine Ylide – A New Example of Multiple C–C‐Bond Forming Transformations.
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-09-10 , DOI: 10.1002/slct.201802117 Alexey M. Starosotnikov 1 , Maxim A. Bastrakov 1 , Vadim V. Kachala 1 , Ivan V. Fedyanin 2 , Svyatoslav A. Shevelev 1 , Igor L. Dalinger 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2018-09-10 , DOI: 10.1002/slct.201802117 Alexey M. Starosotnikov 1 , Maxim A. Bastrakov 1 , Vadim V. Kachala 1 , Ivan V. Fedyanin 2 , Svyatoslav A. Shevelev 1 , Igor L. Dalinger 1
Affiliation
|
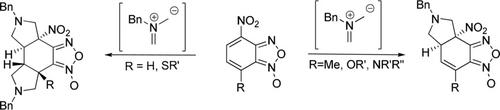
A series of 4‐nitrobenzofuroxans were examined in [3+2]‐cycloaddition reactions with N‐benzyl azomethine ylide. The reaction outcome was found to be dependent on the nature of substituent 7‐R: for R=H and SR’ the products of double cycloaddition of the dipole were isolated, whereas for R=CH3, OR’ and NR'R” tetrahydroisoindoles annelated with furoxan ring were the sole products. As a result efficient chemoselective method for the synthesis of mono‐ and bis‐pyrrolidine fused benzofuroxan derivatives was developed.
中文翻译:

N-苄基甲亚胺叶立德在1,3-偶极环加成反应中4-硝基苯并呋喃类化合物的异常环周反应性–多个C–C–键形成转化的新例子。
在与N-苄基甲亚胺叶立德[3 + 2]-环加成反应中检测了一系列4-硝基苯并呋喃。发现反应结果取决于取代基7-R的性质:对于R = H和SR',分离了偶极的双环加成产物,而对于R = CH 3,OR'和NR'R”是四氢异吲哚唯一的产品是用呋喃喃环退火的产品。因此,开发了一种高效的化学选择性方法,用于合成单吡咯和双吡咯烷稠合的苯并呋喃衍生物。
更新日期:2018-09-10
中文翻译:

N-苄基甲亚胺叶立德在1,3-偶极环加成反应中4-硝基苯并呋喃类化合物的异常环周反应性–多个C–C–键形成转化的新例子。
在与N-苄基甲亚胺叶立德[3 + 2]-环加成反应中检测了一系列4-硝基苯并呋喃。发现反应结果取决于取代基7-R的性质:对于R = H和SR',分离了偶极的双环加成产物,而对于R = CH 3,OR'和NR'R”是四氢异吲哚唯一的产品是用呋喃喃环退火的产品。因此,开发了一种高效的化学选择性方法,用于合成单吡咯和双吡咯烷稠合的苯并呋喃衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号