当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Insights into the Interaction Mechanisms between Nitric Acid and Nitrous Oxide Initiated by an Excess Electron
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-09-11 00:00:00 , DOI: 10.1021/acs.jpca.8b04775 Yun Zhao 1 , Weihua Wang 1 , Wenling Feng 1 , Wenliang Wang 1 , Ping Li 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-09-11 00:00:00 , DOI: 10.1021/acs.jpca.8b04775 Yun Zhao 1 , Weihua Wang 1 , Wenling Feng 1 , Wenliang Wang 1 , Ping Li 1
Affiliation
|
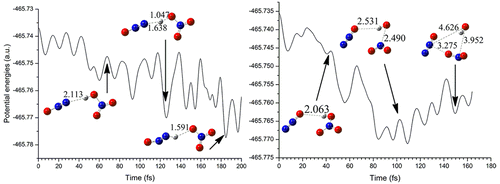
Nitric acid (HNO3) and nitrous oxide (N2O) play an important role in the atmospheric chemistry in regulating the global environment and climate changes. In this study, the interaction mechanisms between them have been systematically investigated before and after the electron capture employing the density functional theory in combination with the AIM, NBO, and ab initio molecular dynamics calculations. It was found that HNO3 and N2O can form transient complexes through intermolecular H-bonds. HNNO, OH, and NO2 free radicals can be produced after the electron capture of the formed complexes, providing an alternative source of these radicals in the atmosphere. The present results not only can provide new insights into the transformation of the HNO3 and N2O atmospheric species but also can enable us to better understand the potential role of the free electron in the atmosphere.
中文翻译:

过量电子引发硝酸与一氧化二氮相互作用机理的理论研究
硝酸(HNO 3)和一氧化二氮(N 2 O)在调节全球环境和气候变化的大气化学中起着重要作用。在这项研究中,已经使用密度泛函理论结合AIM,NBO和从头算分子动力学计算系统地研究了电子捕获前后它们之间的相互作用机理。发现HNO 3和N 2 O可通过分子间的氢键形成瞬态复合物。HNNO,OH和NO 2在电子捕获形成的配合物之后可以产生自由基,为大气中这些自由基提供了另一种来源。目前的结果不仅可以为HNO 3和N 2 O大气物质的转化提供新的见解,还可以使我们更好地了解自由电子在大气中的潜在作用。
更新日期:2018-09-11
中文翻译:

过量电子引发硝酸与一氧化二氮相互作用机理的理论研究
硝酸(HNO 3)和一氧化二氮(N 2 O)在调节全球环境和气候变化的大气化学中起着重要作用。在这项研究中,已经使用密度泛函理论结合AIM,NBO和从头算分子动力学计算系统地研究了电子捕获前后它们之间的相互作用机理。发现HNO 3和N 2 O可通过分子间的氢键形成瞬态复合物。HNNO,OH和NO 2在电子捕获形成的配合物之后可以产生自由基,为大气中这些自由基提供了另一种来源。目前的结果不仅可以为HNO 3和N 2 O大气物质的转化提供新的见解,还可以使我们更好地了解自由电子在大气中的潜在作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号