当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adventures in Atropisomerism: Development of a Robust, Diastereoselective, Lithium-Catalyzed Atropisomer-Forming Active Pharmaceutical Ingredient Step
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-09-11 00:00:00 , DOI: 10.1021/acs.oprd.8b00246 Steven R. Wisniewski 1 , Ronald Carrasquillo-Flores 1 , Federico Lora Gonzalez 1 , Antonio Ramirez 1 , Matthew Casey 1 , Maxime Soumeillant 1 , Thomas M. Razler 1 , Brendan Mack 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-09-11 00:00:00 , DOI: 10.1021/acs.oprd.8b00246 Steven R. Wisniewski 1 , Ronald Carrasquillo-Flores 1 , Federico Lora Gonzalez 1 , Antonio Ramirez 1 , Matthew Casey 1 , Maxime Soumeillant 1 , Thomas M. Razler 1 , Brendan Mack 1
Affiliation
|
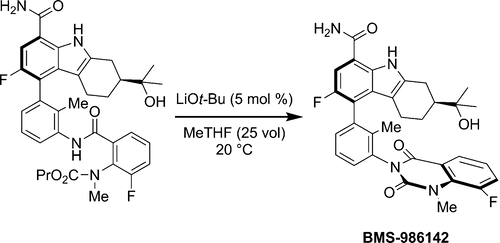
The final step in the route to BMS-986142, a reversible inhibitor of the BTK enzyme, involves the diastereoselective construction of a chiral axis during the base-mediated cyclization of the quinazolinedione fragment. Optimization of the reaction to minimize formation of the undesired atropisomer led to the discovery that the amount of base and nature of the counterion play a vital role in the diastereoselectivity of the reaction. The highest diastereoselectivities were observed with a catalytic amount of LiOt-Bu. Development of a crystallization to selectively purge the undesired atropisomer is reported. Interestingly, ripening of the crystalline API was observed and further investigated, leading to a significant increase in the purity of the active pharmaceutical ingredient.
中文翻译:

Atropisomerism历险记:鲁棒的,非对映选择性,锂催化的Atropisomer形成活性药物成分步骤的发展。
通往BMS-986142(一种BTK酶的可逆抑制剂)的最后一步涉及在喹唑啉二酮片段的碱基介导环化过程中手性轴的非对映选择性构建。优化反应以最小化不希望的阻转异构体的形成导致发现,抗衡离子的碱的量和性质在反应的非对映选择性中起着至关重要的作用。催化量的LiO t -Bu观察到最高的非对映选择性。据报道结晶的发展以选择性地清除不希望的阻转异构体。有趣的是,观察到并进一步研究了结晶API的成熟,从而导致活性药物成分纯度的显着提高。
更新日期:2018-09-11
中文翻译:

Atropisomerism历险记:鲁棒的,非对映选择性,锂催化的Atropisomer形成活性药物成分步骤的发展。
通往BMS-986142(一种BTK酶的可逆抑制剂)的最后一步涉及在喹唑啉二酮片段的碱基介导环化过程中手性轴的非对映选择性构建。优化反应以最小化不希望的阻转异构体的形成导致发现,抗衡离子的碱的量和性质在反应的非对映选择性中起着至关重要的作用。催化量的LiO t -Bu观察到最高的非对映选择性。据报道结晶的发展以选择性地清除不希望的阻转异构体。有趣的是,观察到并进一步研究了结晶API的成熟,从而导致活性药物成分纯度的显着提高。









































 京公网安备 11010802027423号
京公网安备 11010802027423号