Journal of Power Sources ( IF 8.1 ) Pub Date : 2018-09-10 , DOI: 10.1016/j.jpowsour.2018.09.002 Pedro Berastegui , Cheuk-Wai Tai , Mario Valvo
|
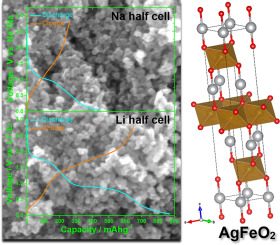
AgFeO2 nanoparticles synthesized via precipitation at room temperature are investigated in Li- and Na-ion cells through electrode coatings with an alginate binder. The electrochemical reactions of AgFeO2 with Li+ and Na+ ions, as well as its role as alternative negative electrode in these cell systems are carefully evaluated. Initial Li uptake causes irreversible amorphization of the AgFeO2 structure with concomitant formation of Ag0 nanoparticles. Further Li incorporation results in conversion into Fe0 nanoparticles and Li2O, together with Li-alloying of these Ag0 clusters. Similar mechanisms are also found upon Na uptake, although such processes are hindered by overpotentials, the capacity and reversibility of the reactions with Na+ ions being not comparable with those of their Li+ counterparts. The behaviour of AgFeO2 at low potentials vs. Li+/Li displays a synergic pseudo-capacitive charge storage overlapping Li-Ag alloying/de-alloying. This feature is exploited in full cells having deeply lithiated AgFeO2 and LiFePO4 as negative and positive electrodes, respectively. These environmentally friendly iron-based full cells exhibit attractive cycle performances with ≈80% capacity retention after 1000 cycles without any electrolyte additive, average round trip efficiency of ≈89% and operational voltage of 3.0 V combined with built-in pseudo-capacitive characteristics that enable high cycling rates up to ≈25C.
中文翻译:

锂和钠离子电池中AgFeO 2作为负极的电化学反应
通过使用藻酸盐粘合剂的电极涂层,在锂离子和钠离子电池中研究了在室温下通过沉淀合成的AgFeO 2纳米颗粒。仔细评估了AgFeO 2与Li +和Na +离子的电化学反应,以及其在这些电池系统中作为替代负极的作用。最初的Li吸收会导致AgFeO 2结构不可逆地非晶化,并同时形成Ag 0纳米颗粒。进一步的Li掺入导致转化为Fe 0纳米颗粒和Li 2 O,以及这些Ag 0的锂合金化集群。钠的吸收也发现了类似的机理,尽管这种过程受过电势的阻碍,与Na +离子反应的能力和可逆性与Li +对应物的反应能力和可逆性不相上下。AgFeO 2在低电势下(相对于Li + / Li的行为)显示出与Li-Ag合金化/脱合金重叠的协同伪电容电荷存储。在具有深锂化的AgFeO 2和LiFePO 4的完整电池中利用了此功能分别作为负极和正极。这些环保的铁基全电池在无任何电解质添加剂的情况下,经过1000次循环后,仍具有有吸引力的循环性能,≈80%的容量保持率,平均往返效率≈89%,工作电压3.0 V,以及内置的伪电容特性实现高达≈25C的高循环速率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号