当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Boron(III)‐Catalyzed C2‐Selective C−H Borylation of Heteroarenes
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-10-12 , DOI: 10.1002/anie.201808590 Qi Zhong 1 , Shengxiang Qin 1 , Youzhi Yin 1 , Jiajun Hu 1 , Hua Zhang 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-10-12 , DOI: 10.1002/anie.201808590 Qi Zhong 1 , Shengxiang Qin 1 , Youzhi Yin 1 , Jiajun Hu 1 , Hua Zhang 1
Affiliation
|
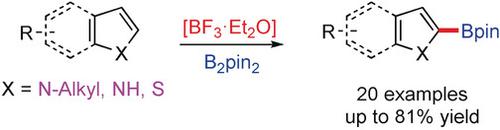
A BF3⋅Et2O‐catalyzed C2‐selective C−H borylation of indoles with bis(pinacolato)diboron was developed to afford indole‐2‐boronic acid pinacol esters. A variety of functional groups were tolerated, and other heteroarenes like pyrrole and benzo[b]thiophene were also suitable substrates. An electrophilic substitution mechanism was proposed based on the preliminary mechanistic studies. This novel transformation utilizes simple and cheap BF3⋅Et2O as catalyst and exhibits unusual C2 regioselectivity, providing a significant non‐transition‐metal‐catalyzed C−H borylation and an efficient method towards the synthesis of C2‐functionalized heteroarenes.
中文翻译:

硼(III)催化杂芳烃的C2选择性CH硼化
甲BF 3 ⋅Et 2 O形催化C2选择性C-H与双(频哪醇合)二硼的吲哚的硼基化的开发是为了得到吲哚-2-硼酸频哪醇酯。可以容忍各种官能团,其他杂芳烃如吡咯和苯并[ b ]噻吩也是合适的底物。在初步的机理研究的基础上,提出了一种亲电取代机理。这种新颖的变换利用简单和便宜的BF 3 ⋅Et 2 O作为催化剂,表现出不寻常的C2的区域选择性,提供了显著非过渡金属催化C-H硼化并朝向C2官能杂芳烃的合成的有效方法。
更新日期:2018-10-12
中文翻译:

硼(III)催化杂芳烃的C2选择性CH硼化
甲BF 3 ⋅Et 2 O形催化C2选择性C-H与双(频哪醇合)二硼的吲哚的硼基化的开发是为了得到吲哚-2-硼酸频哪醇酯。可以容忍各种官能团,其他杂芳烃如吡咯和苯并[ b ]噻吩也是合适的底物。在初步的机理研究的基础上,提出了一种亲电取代机理。这种新颖的变换利用简单和便宜的BF 3 ⋅Et 2 O作为催化剂,表现出不寻常的C2的区域选择性,提供了显著非过渡金属催化C-H硼化并朝向C2官能杂芳烃的合成的有效方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号