当前位置:
X-MOL 学术
›
Biomaterials
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stiffness memory of indirectly 3D-printed elastomer nanohybrid regulates chondrogenesis and osteogenesis of human mesenchymal stem cells.
Biomaterials ( IF 12.8 ) Pub Date : 2018-09-10 , DOI: 10.1016/j.biomaterials.2018.09.013 Linxiao Wu 1 , Adrián Magaz 1 , Tao Wang 2 , Chaozong Liu 3 , Arnold Darbyshire 1 , Marilena Loizidou 1 , Mark Emberton 1 , Martin Birchall 4 , Wenhui Song 1
Biomaterials ( IF 12.8 ) Pub Date : 2018-09-10 , DOI: 10.1016/j.biomaterials.2018.09.013 Linxiao Wu 1 , Adrián Magaz 1 , Tao Wang 2 , Chaozong Liu 3 , Arnold Darbyshire 1 , Marilena Loizidou 1 , Mark Emberton 1 , Martin Birchall 4 , Wenhui Song 1
Affiliation
|
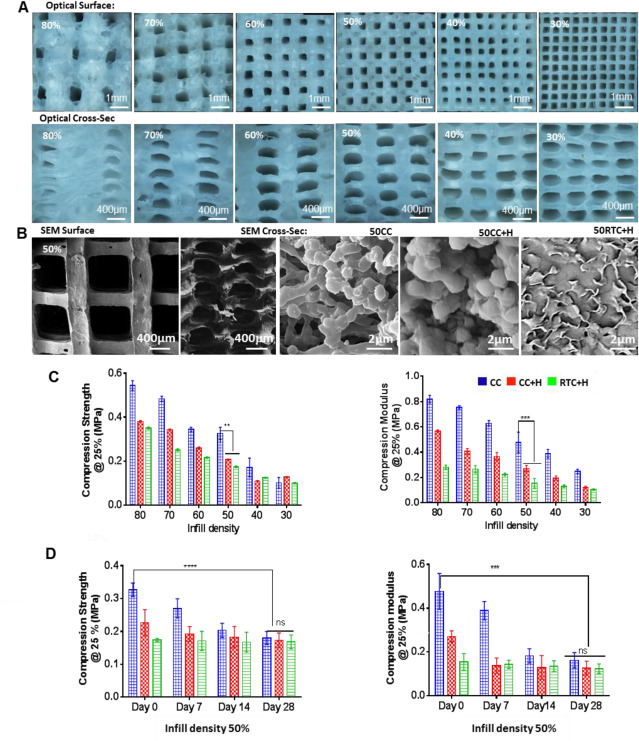
The cellular microenvironment is dynamic, remodeling tissues lifelong. The biomechanical properties of the extracellular matrix (ECM) influence the function and differentiation of stem cells. While conventional artificial matrices or scaffolds for tissue engineering are primarily static models presenting well-defined stiffness, they lack the responsive changes required in dynamic physiological settings. Engineering scaffolds with varying elastic moduli is possible, but often lead to stiffening and chemical crosslinking of the molecular structure with limited control over the scaffold architecture. A family of indirectly 3D printed elastomeric nanohybrid scaffolds with thermoresponsive mechanical properties that soften by reverse self-assembling at body temperature have been developed recently. The initial stiffness and subsequent stiffness relaxation of the scaffolds regulated proliferation and differentiation of human bone-marrow derived mesenchymal stem cells (hBM-MSCs) towards the chondrogenic and osteogenic lineages over 4 weeks, as measured by immunohistochemistry, histology, ELISA and qPCR. hBM-MSCs showed enhanced chondrogenic differentiation on softer scaffolds and osteogenic differentiation on stiffer ones, with similar relative expression to that of human femoral head tissue. Overall, stiffness relaxation favored osteogenic activity over chondrogenesis in vitro.
中文翻译:

间接3D打印的弹性体nanohybrid的刚度记忆可调节人间充质干细胞的软骨形成和成骨作用。
细胞微环境是动态的,可重生组织。细胞外基质(ECM)的生物力学特性影响干细胞的功能和分化。尽管用于组织工程的常规人工基质或支架主要是具有明确定义的刚度的静态模型,但它们缺乏动态生理环境中所需的响应性变化。具有变化的弹性模量的工程支架是可能的,但是常常导致分子结构的硬化和化学交联,而对支架结构的控制有限。最近已经开发了一系列间接3D打印的弹性体纳米杂化支架,这些支架具有在人体温度下通过逆向自组装而软化的热响应机械性能。支架的初始刚度和随后的刚度松弛调节了人类骨髓衍生的间充质干细胞(hBM-MSC)在4周内向软骨形成和成骨细胞谱系的增殖和分化,这通过免疫组织化学,组织学,ELISA和qPCR进行测量。hBM-MSC在较软的支架上显示出增强的软骨形成分化,而在较硬的支架上显示出成骨分化,其相对表达与人股骨头组织相似。总体而言,刚度松弛比体外软骨形成更有利于成骨活性。hBM-MSC在较软的支架上显示出增强的软骨分化,在较硬的支架上显示出成骨分化,其相对表达与人股骨头组织相似。总体而言,刚度松弛比体外软骨形成更有利于成骨活性。hBM-MSC在较软的支架上显示出增强的软骨形成分化,而在较硬的支架上显示出成骨分化,其相对表达与人股骨头组织相似。总体而言,刚度松弛比体外软骨形成更有利于成骨活性。
更新日期:2018-09-11
中文翻译:

间接3D打印的弹性体nanohybrid的刚度记忆可调节人间充质干细胞的软骨形成和成骨作用。
细胞微环境是动态的,可重生组织。细胞外基质(ECM)的生物力学特性影响干细胞的功能和分化。尽管用于组织工程的常规人工基质或支架主要是具有明确定义的刚度的静态模型,但它们缺乏动态生理环境中所需的响应性变化。具有变化的弹性模量的工程支架是可能的,但是常常导致分子结构的硬化和化学交联,而对支架结构的控制有限。最近已经开发了一系列间接3D打印的弹性体纳米杂化支架,这些支架具有在人体温度下通过逆向自组装而软化的热响应机械性能。支架的初始刚度和随后的刚度松弛调节了人类骨髓衍生的间充质干细胞(hBM-MSC)在4周内向软骨形成和成骨细胞谱系的增殖和分化,这通过免疫组织化学,组织学,ELISA和qPCR进行测量。hBM-MSC在较软的支架上显示出增强的软骨形成分化,而在较硬的支架上显示出成骨分化,其相对表达与人股骨头组织相似。总体而言,刚度松弛比体外软骨形成更有利于成骨活性。hBM-MSC在较软的支架上显示出增强的软骨分化,在较硬的支架上显示出成骨分化,其相对表达与人股骨头组织相似。总体而言,刚度松弛比体外软骨形成更有利于成骨活性。hBM-MSC在较软的支架上显示出增强的软骨形成分化,而在较硬的支架上显示出成骨分化,其相对表达与人股骨头组织相似。总体而言,刚度松弛比体外软骨形成更有利于成骨活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号