当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Where and How Does an Organic Molecule Having a C–X Bond Release X– Anion Like an Inorganic Compound? A Theoretical Study
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-09-10 00:00:00 , DOI: 10.1021/acs.jpca.8b07238 Sadegh Salehzadeh 1 , Farahnaz Maleki 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-09-10 00:00:00 , DOI: 10.1021/acs.jpca.8b07238 Sadegh Salehzadeh 1 , Farahnaz Maleki 1
Affiliation
|
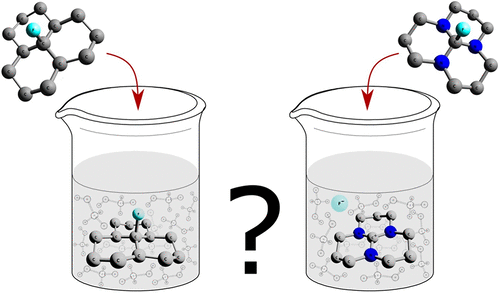
In this research, at first, a comparative DFT study on hydride or fluoride release from a number of known orthoamides, their fluorine derivative having a central C–F bond and some simple organic compounds, is reported. The obtained data show that orthoamides release hydride or fluoride anions much easier than do other known organic compounds studied here. Interestingly, three simulated orthoamides having a central C–F bond, spontaneously and like an inorganic compound, release fluoride anion upon dissolving in polar solvents. The calculations confirmed that hyperconjugation interactions in the initial orthoamides facilitate the anion release from these compounds. However, the data clearly show that the proper overlap of an empty p orbital of the central carbon atom with adjacent lone-pair orbitals of nitrogen atoms (lpN → lp*C or, in other words, the pπ–pπ interaction) in the resulting carbocations is a more important factor that must be taken into consideration when designing the hydride, fluoride, or other anion releasing agents. In addition, for the first time, a mechanism of hydride and fluoride removal from the orthoamides either through their reaction with BH3 and BF3 molecules, respectively, or upon their protonation is provided. To continue and for generalization of results to other groups attached to the central carbon atom of orthoamides, the reactions of H+ with orthoamides having the CH3, OH, CN, NH2, or C5H5 substituents were studied. The results showed that in all cases and in both gas and solution phases the above substituents easily leave the carbon atom as an anion and bond to H+. Thus, we have to conclude that upon the reaction of orthoamides with usual Lewis acids, many types of substituents on their central carbon atom can leave these compounds as anions.
中文翻译:

具有C–X键的有机分子将在何处以及如何像无机化合物一样释放X –阴离子?理论研究
在这项研究中,首先,对DFT进行了比较研究,该研究涉及从许多已知的原酰胺,其具有中心C-F键的氟衍生物和一些简单的有机化合物中释放出氢化物或氟化物的报道。获得的数据表明,与本文研究的其他已知有机化合物相比,原酰胺更容易释放氢化物或氟化物阴离子。有趣的是,三种模拟的具有中心C-F键的邻位酰胺自发地像无机化合物一样,溶于极性溶剂后会释放出氟离子。该计算证实了初始邻酰胺中的超共轭相互作用促进了阴离子从这些化合物中的释放。但是,数据清楚地表明,中心碳原子的空p轨道与相邻的氮原子孤对轨道有适当的重叠(lp在设计氢化物,氟化物或其他阴离子释放剂时,必须考虑到N→ lp * C或换言之,碳正离子中的pπ–pπ相互作用)。另外,首次提供了通过分别与BH 3和BF 3分子反应或在其质子化时从邻酰胺中除去氢化物和氟化物的机理。为了将结果继续推广到与邻酰胺的中心碳原子相连的其他基团上,H +与具有CH 3,OH,CN,NH 2或C 5 H 5的邻酰胺的反应研究了取代基。结果表明,在所有情况下,无论在气相还是在溶液相中,上述取代基都容易留下作为阴离子的碳原子并与H +结合。因此,我们必须得出结论,在原酰胺与常用的路易斯酸反应后,其中心碳原子上的许多类型取代基可将这些化合物保留为阴离子。
更新日期:2018-09-10
中文翻译:

具有C–X键的有机分子将在何处以及如何像无机化合物一样释放X –阴离子?理论研究
在这项研究中,首先,对DFT进行了比较研究,该研究涉及从许多已知的原酰胺,其具有中心C-F键的氟衍生物和一些简单的有机化合物中释放出氢化物或氟化物的报道。获得的数据表明,与本文研究的其他已知有机化合物相比,原酰胺更容易释放氢化物或氟化物阴离子。有趣的是,三种模拟的具有中心C-F键的邻位酰胺自发地像无机化合物一样,溶于极性溶剂后会释放出氟离子。该计算证实了初始邻酰胺中的超共轭相互作用促进了阴离子从这些化合物中的释放。但是,数据清楚地表明,中心碳原子的空p轨道与相邻的氮原子孤对轨道有适当的重叠(lp在设计氢化物,氟化物或其他阴离子释放剂时,必须考虑到N→ lp * C或换言之,碳正离子中的pπ–pπ相互作用)。另外,首次提供了通过分别与BH 3和BF 3分子反应或在其质子化时从邻酰胺中除去氢化物和氟化物的机理。为了将结果继续推广到与邻酰胺的中心碳原子相连的其他基团上,H +与具有CH 3,OH,CN,NH 2或C 5 H 5的邻酰胺的反应研究了取代基。结果表明,在所有情况下,无论在气相还是在溶液相中,上述取代基都容易留下作为阴离子的碳原子并与H +结合。因此,我们必须得出结论,在原酰胺与常用的路易斯酸反应后,其中心碳原子上的许多类型取代基可将这些化合物保留为阴离子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号