Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2018-09-10 , DOI: 10.1016/j.bmcl.2018.09.011 James T. Fletcher , Jill M. Sobczyk , Sarah C. Gwazdacz , Aaron J. Blanck
|
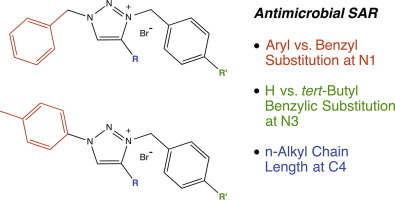
A series of 1,3,4-trisubstituted-1,2,3-triazolium bromide salts were prepared by efficient two-step sequences of azide-alkyne cycloaddition and benzylic substitution. The antimicrobial activity of each triazolium salt and correlating triazole precursor was evaluated using a minimum inhibitory concentration (MIC) assay. MIC activities as low as 1 µM against Gram-positive bacteria, 8 µM against Gram-negative bacteria and 4 µM against fungi were observed for salt analogs, while neutral triazoles were inactive. Analogs representing selective and broad-spectrum antimicrobial activity were each identified. MIC structure-activity relationships observed within this motif indicate that the presence of cationic charge and balance of overall hydrophobicity are strongly impactful, while benzyl vs. aryl substituent identity and variation of substituent regiochemistry are not.
中文翻译:

抗菌1,3,4-三取代-1,2,3-三唑鎓盐
通过叠氮化物-炔烃环加成和苄基取代的有效两步序列,制备了一系列1,3,4-三取代-1,2,3-三唑鎓溴化物盐。使用最小抑菌浓度(MIC)分析评估每种三唑鎓盐和相关的三唑前体的抗菌活性。盐类似物对革兰氏阳性菌的MIC活性低至1 µM,对革兰氏阴性菌的MIC活性低至4 µM,而对中性三唑则无活性。分别鉴定了代表选择性和广谱抗菌活性的类似物。在此基序中观察到的MIC结构与活性的关系表明,阳离子电荷的存在和整体疏水性的平衡具有强烈的影响力,而苄基vs.











































 京公网安备 11010802027423号
京公网安备 11010802027423号