当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pt/TS-1 Catalyst Promoted C–N Coupling Reaction in CH4–NH3 Plasma for HCN Synthesis at Low Temperature
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-09-10 00:00:00 , DOI: 10.1021/acscatal.8b02950 Zhifang Guo 1 , Yanhui Yi 1 , Li Wang 2 , Jinhui Yan 1 , Hongchen Guo 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-09-10 00:00:00 , DOI: 10.1021/acscatal.8b02950 Zhifang Guo 1 , Yanhui Yi 1 , Li Wang 2 , Jinhui Yan 1 , Hongchen Guo 1
Affiliation
|
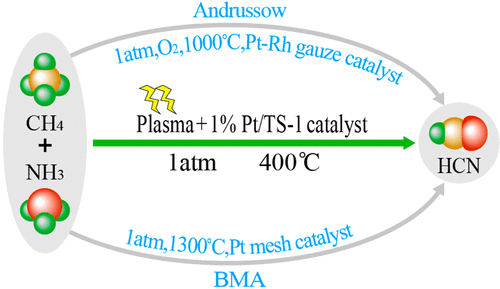
Hydrocyanic acid is an important synthetic material in chemical industry. Currently, the industrial methods of producing HCN mainly include the Andrussow and BMA processes, which are necessarily operated at temperatures of more than 1000 °C. This paper proposes a method to synthesize HCN at low temperature (400 °C) through a C–N coupling reaction in CH4/NH3 nonthermal plasma with the assistance of Pt/TS-1 catalyst. Under the optimized reaction conditions (CH4/NH3 = 1:2, GHSV: 2425 s–1, discharge length: 3 cm, reaction temperature: 400 °C, Pt/TS-1 + plasma), 80.9% HCN selectivity was achieved with 25.9% CH4 conversion. During the reaction process, the nonthermal plasma played a role of activating CH4 and NH3 into radical species (CHx and NHy), while the Pt/TS-1 catalyst promoted the C–N coupling reaction of the radical species to produce HCN.
中文翻译:

Pt / TS-1催化剂在CH 4 -NH 3等离子体中促进C–N偶联反应,用于低温合成HCN
氢氰酸是化学工业中重要的合成材料。当前,生产HCN的工业方法主要包括Andrussow和BMA工艺,它们必须在高于1000°C的温度下操作。本文提出了一种在Pt / TS-1催化剂的辅助下,在CH 4 / NH 3非热等离子体中通过C–N偶联反应在低温(400°C)下合成HCN的方法。在优化的反应条件下(CH 4 / NH 3 = 1:2,GHSV:2425 s –1,放电长度:3 cm,反应温度:400°C,Pt / TS-1 +等离子体),HCN选择性为80.9%用25.9%CH 4达到转换。在反应过程中,非热等离子体起到了将CH 4和NH 3活化成自由基物质(CH x和NH y)的作用,而Pt / TS-1催化剂促进了自由基物质的C–N偶联反应,从而产生了自由基。 HCN。
更新日期:2018-09-10
中文翻译:

Pt / TS-1催化剂在CH 4 -NH 3等离子体中促进C–N偶联反应,用于低温合成HCN
氢氰酸是化学工业中重要的合成材料。当前,生产HCN的工业方法主要包括Andrussow和BMA工艺,它们必须在高于1000°C的温度下操作。本文提出了一种在Pt / TS-1催化剂的辅助下,在CH 4 / NH 3非热等离子体中通过C–N偶联反应在低温(400°C)下合成HCN的方法。在优化的反应条件下(CH 4 / NH 3 = 1:2,GHSV:2425 s –1,放电长度:3 cm,反应温度:400°C,Pt / TS-1 +等离子体),HCN选择性为80.9%用25.9%CH 4达到转换。在反应过程中,非热等离子体起到了将CH 4和NH 3活化成自由基物质(CH x和NH y)的作用,而Pt / TS-1催化剂促进了自由基物质的C–N偶联反应,从而产生了自由基。 HCN。











































 京公网安备 11010802027423号
京公网安备 11010802027423号