当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
MicroPlate Sialyltransferase Assay: A Rapid and Sensitive Assay Based on an Unnatural Sialic Acid Donor and Bioorthogonal Chemistry
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-09-07 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00529 Maxence Noel 1 , Pierre-André Gilormini 1 , Virginie Cogez 1 , Cédric Lion 1 , Christophe Biot 1 , Anne Harduin-Lepers 1 , Yann Guérardel 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-09-07 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00529 Maxence Noel 1 , Pierre-André Gilormini 1 , Virginie Cogez 1 , Cédric Lion 1 , Christophe Biot 1 , Anne Harduin-Lepers 1 , Yann Guérardel 1
Affiliation
|
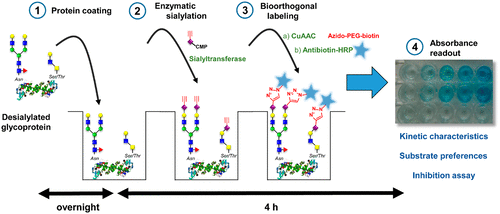
Mammalian sialyltransferases transfer sialic acids onto glycoproteins and glycolipids within the Golgi apparatus. Despite their key role in glycosylation, the study of their enzymatic activities is limited by the lack of appropriate tools. Herein, we developed a quick and sensitive sialyltransferase microplate assay based on the use of the unnatural CMP-SiaNAl donor substrate. In this assay, an appropriate acceptor glycoprotein is coated on the bottom of 96-well plate and the sialyltransferase activity is assessed using CMP-SiaNAl. The alkyne tag of SiaNAl enables subsequent covalent ligation of an azido-biotin probe via CuAAC and an antibiotin-HRP conjugated antibody is then used to quantify the amount of transferred SiaNAl by a colorimetric titration. With this test, we evaluated the kinetic characteristics and substrate preferences of two human sialyltransferases, ST6Gal I and ST3Gal I toward a panel of asialoglycoprotein acceptors, and identified cations that display a sialyltransferase inhibitory effect.
中文翻译:

MicroPlate唾液酸转移酶测定:基于非天然唾液酸供体和生物正交化学的快速灵敏测定
哺乳动物的唾液酸转移酶将唾液酸转移到高尔基体中的糖蛋白和糖脂上。尽管它们在糖基化中起关键作用,但由于缺乏合适的工具,其酶活性的研究受到了限制。本文中,我们基于非天然CMP-SiaNAl供体底物的使用,开发了一种快速灵敏的唾液酸转移酶微孔板测定法。在该测定中,将合适的受体糖蛋白包被在96孔板的底部,并使用CMP-SiaNA1评估唾液酸转移酶的活性。SiaNA1的炔烃标记可通过CuAAC进行叠氮基-生物素探针的后续共价连接,然后将抗生物素-HRP偶联抗体用于通过比色滴定法定量SiaNA1的转移量。有了这个测试,
更新日期:2018-09-07
中文翻译:

MicroPlate唾液酸转移酶测定:基于非天然唾液酸供体和生物正交化学的快速灵敏测定
哺乳动物的唾液酸转移酶将唾液酸转移到高尔基体中的糖蛋白和糖脂上。尽管它们在糖基化中起关键作用,但由于缺乏合适的工具,其酶活性的研究受到了限制。本文中,我们基于非天然CMP-SiaNAl供体底物的使用,开发了一种快速灵敏的唾液酸转移酶微孔板测定法。在该测定中,将合适的受体糖蛋白包被在96孔板的底部,并使用CMP-SiaNA1评估唾液酸转移酶的活性。SiaNA1的炔烃标记可通过CuAAC进行叠氮基-生物素探针的后续共价连接,然后将抗生物素-HRP偶联抗体用于通过比色滴定法定量SiaNA1的转移量。有了这个测试,











































 京公网安备 11010802027423号
京公网安备 11010802027423号