Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-09-08 , DOI: 10.1016/j.actbio.2018.09.005 Noorjahan Aibani , Heather Nesbitt , Nino Marino , Joanna Jurek , Caolin O'Neill , Chloe Martin , Ivana Di Bari , Yingjie Sheng , Kieran Logan , Susan Hawthorne , Anthony McHale , John F. Callan , Bridgeen Callan
|
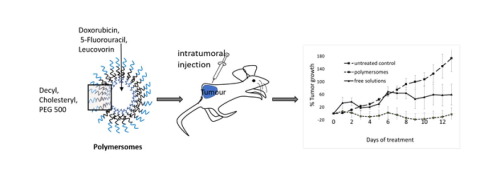
Combination cancer chemotherapy provides an important treatment tool, both as an adjuvant and neoadjuvant treatment, this shift in focus from mono to combination therapies has led to increased interest in drug delivery systems (DDS). DDSs, such as polymersomes, are capable of encapsulating large amounts of multiple drugs with both hydrophilic and hydrophobic properties simultaneously, as well as offering a mechanism to combat multi drug resistant cancers and poor patient tolerance of the cytotoxic compounds utilised. In this article, we report the formulation and evaluation of a novel electroneutral polymersome capable of high encapsulation efficacies for multiple drugs (Doxorubicin, 5-Fluorouracil and leucovorin). The in-vivo biodistribution of the polymersome were established and they were found to accumulate largely in tumour tissue. Polymersome encapsulating the three chemotherapeutic drugs were assessed both in-vitro (BxPC-3 cell line) and in-vivo (following intratumoral and intravenous administration) and compared with the same concentration of the three drugs in solution. We report better efficacy and higher maximum tolerated dose for our combination drug loaded polymersomes in all experiments. Furthermore, intratumorally injected combination drug loaded polymersomes exhibited a 62% reduction in tumour volume after 13 days when compared with the free combination solutions. A smaller differential of 13% was observed for when treatment was administered intravenously however, importantly less cardiotoxicity was displayed from the polymersomal DDS. In this study, expression of a number of survival-relevant genes in tumours treated with the free chemotherapy combination was compared with expression of those genes in tumours treated with the polymersomes harbouring those drugs and the significance of findings is discussed.
Statement of significance
The shift in focus from mono to combination chemotherapies has led to an increased interest in the role of drug delivery systems (DDS). Liposomes, although commercialized for mono therapy, have lower loading capacities and stability than their polymeric counterpart, polymersomes. Polymersomes are growing in prevalence as their advantageous properties are better understood and exploited. Here we present a novel polymersome for the encapsulation of three anticancer compounds. This is the first time this particular polymersome has been used to encapsulate these three compounds with both an in-vitro and in-vivo evaluation carried out. This work will be of interest to those in the field of combination therapy, drug delivery, drug toxicity, multidrug resistance, liposomes, DDS and polymersomes.
中文翻译:

电中性聚合物囊泡用于联合癌症化疗
组合癌化学疗法提供了重要的治疗工具,无论是辅助治疗还是新辅助治疗,焦点从单一疗法转移到组合疗法都引起了人们对药物递送系统(DDS)的日益增长的兴趣。DDS,例如聚合物囊泡,能够同时封装大量具有亲水性和疏水性的多种药物,并提供了对抗多种耐药性癌症和患者对所利用的细胞毒性化合物的不良耐受性的机制。在本文中,我们报告了一种新型的电子中性聚合物囊泡的配方和评价,该囊泡对多种药物(阿霉素,5-氟尿嘧啶和亚叶酸钙蛋白)具有很高的包封效率。在体内建立了聚合物囊泡的生物分布,发现它们在肿瘤组织中大量积累。封装了三种化疗药物的聚合物囊泡在体外(BxPC-3细胞系)和体内进行了评估(在肿瘤内和静脉内给药之后),并与相同浓度的三种药物在溶液中进行比较。我们报告了在所有实验中,我们的组合药物负载聚合物囊泡的疗效更好,最大耐受剂量更高。此外,与游离组合溶液相比,肿瘤内注射的组合药物负载的聚合物囊泡在13天后显示出62%的肿瘤体积减小。静脉内给予治疗时观察到较小的13%差异,但是重要的是,多聚体DDS显示出较小的心脏毒性。在这项研究中,
重要声明
从单一化学疗法到复合化学疗法的关注点转移,引起了人们对药物输送系统(DDS)作用的越来越浓厚的兴趣。脂质体尽管商业化用于单一疗法,但其负载能力和稳定性却低于其聚合物对应物,聚合物脂质体。随着人们对聚合物小体的有利特性的更好的理解和利用,聚合物小体的流行也在增加。在这里,我们提出了一种新型的聚合物囊泡,用于封装三种抗癌化合物。这是首次通过体外和体内评估,使用这种特殊的聚合物囊体封装这三种化合物。这项工作将对联合治疗,药物递送,药物毒性,多药耐药性,脂质体,DDS和聚合物小体领域的人们感兴趣。











































 京公网安备 11010802027423号
京公网安备 11010802027423号