当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study on MNO3/NO2 (M = Li, Na, and K)/MgO Composites for Intermediate-Temperature CO2 Capture
Energy & Fuels ( IF 5.2 ) Pub Date : 2018-09-07 00:00:00 , DOI: 10.1021/acs.energyfuels.8b02749 Wanlin Gao 1 , Tuantuan Zhou 1 , Yanshan Gao 1 , Qiang Wang 1 , Weiran Lin 2
Energy & Fuels ( IF 5.2 ) Pub Date : 2018-09-07 00:00:00 , DOI: 10.1021/acs.energyfuels.8b02749 Wanlin Gao 1 , Tuantuan Zhou 1 , Yanshan Gao 1 , Qiang Wang 1 , Weiran Lin 2
Affiliation
|
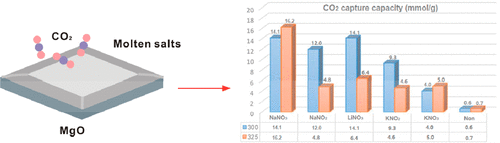
Molten salt [MNO3/NO2 (M = Li, Na, and K)]-promoted MgO composite-type CO2 adsorbents with high capacity and cycling stability are fabricated by controlled hydrolysis along with an incipient impregnation approach. The remarkable results of molten salts on the CO2 capture performance of MgO adsorbents and the CO2 capture capacities with different loadings and calcination and adsorption temperatures were comprehensively investigated. Notably, optimized NaNO3-promoted MgO exhibited the highest CO2 capture capacity of 17.0 mmol g–1 at 350 °C and maintained excellent regenerability through multiple cycles. The initial CO2 uptakes of MNO3/NO2 (M = Li, Na, and K)/MgO composites follow the order of NaNO3 (17.0 mmol g–1) > LiNO3 (14.1 mmol g–1) > NaNO2 (12.5 mmol g–1) > KNO2 (9.3 mmol g–1) > KNO3 (5.0 mmol g–1). Furthermore, NaNO3 and KNO3 with good chemical stability are the effective constituents for the NaNO3 system and KNO3/KNO2-promoted system, respectively, and preserved their original form after the interaction with CO2. The NaNO2 system consists of NaNO3 and Na2CO3 to form double carbonates Na2Mg(CO3)2 after CO2 adsorption. The morphology and structure of the MgO skeleton and the introduction of promoters are essential factors for intermediate-temperature CO2 capture.
中文翻译:

用于中温CO 2捕集的MNO 3 / NO 2(M = Li,Na和K)/ MgO复合材料的研究
熔融盐[MNO 3 / NO 2(M = Li,Na和K)]促进的MgO复合型CO 2吸附剂具有高容量和循环稳定性,是通过控制水解和初期浸渍方法制得的。综合研究了熔盐对MgO吸附剂的CO 2捕集性能和不同负载量,煅烧和吸附温度下的CO 2捕集能力的显着结果。值得注意的是,经优化的NaNO 3促进的MgO在350°C时表现出最高的CO 2捕集能力,为17.0 mmol g –1,并在多个循环中保持出色的可再生性。初始CO 2MNO 3 / NO 2(M = Li,Na和K)/ MgO复合材料的吸收遵循NaNO 3(17.0 mmol g –1)> LiNO 3(14.1 mmol g –1)> NaNO 2(12.5 mmol g )的顺序–1)> KNO 2(9.3 mmol g –1)> KNO 3(5.0 mmol g –1)。此外,纳米3和KNO 3具有良好的化学稳定性是有效成分为纳米3系统和KNO 3 / KNO 2促进体系,并在与CO 2相互作用后保留其原始形式。NaNO 2系统由NaNO 3和Na 2 CO 3组成,在CO 2吸附后形成双碳酸盐Na 2 Mg(CO 3)2。MgO骨架的形态和结构以及促进剂的引入是中温CO 2捕获的必要因素。
更新日期:2018-09-07
中文翻译:

用于中温CO 2捕集的MNO 3 / NO 2(M = Li,Na和K)/ MgO复合材料的研究
熔融盐[MNO 3 / NO 2(M = Li,Na和K)]促进的MgO复合型CO 2吸附剂具有高容量和循环稳定性,是通过控制水解和初期浸渍方法制得的。综合研究了熔盐对MgO吸附剂的CO 2捕集性能和不同负载量,煅烧和吸附温度下的CO 2捕集能力的显着结果。值得注意的是,经优化的NaNO 3促进的MgO在350°C时表现出最高的CO 2捕集能力,为17.0 mmol g –1,并在多个循环中保持出色的可再生性。初始CO 2MNO 3 / NO 2(M = Li,Na和K)/ MgO复合材料的吸收遵循NaNO 3(17.0 mmol g –1)> LiNO 3(14.1 mmol g –1)> NaNO 2(12.5 mmol g )的顺序–1)> KNO 2(9.3 mmol g –1)> KNO 3(5.0 mmol g –1)。此外,纳米3和KNO 3具有良好的化学稳定性是有效成分为纳米3系统和KNO 3 / KNO 2促进体系,并在与CO 2相互作用后保留其原始形式。NaNO 2系统由NaNO 3和Na 2 CO 3组成,在CO 2吸附后形成双碳酸盐Na 2 Mg(CO 3)2。MgO骨架的形态和结构以及促进剂的引入是中温CO 2捕获的必要因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号