当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Approximate Equation To Calculate Partial Pressures in a Mixture of Real Gases
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2018-09-06 00:00:00 , DOI: 10.1021/acs.jchemed.8b00185 Bernard Hayez 1
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2018-09-06 00:00:00 , DOI: 10.1021/acs.jchemed.8b00185 Bernard Hayez 1
Affiliation
|
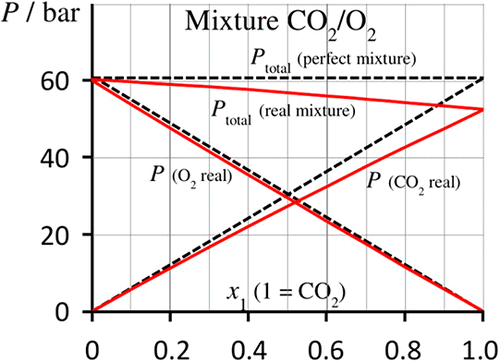
From a physical point of view, the partial pressure of a component i in a gas mixture is defined as the contribution of molecules i to the force per unit area that the molecules of the mixture exert on the wall. The relation pi = xiP (where xi is the mole fraction of i and P the total pressure) is in agreement with this definition for perfect gases, but not for real gases. For lack of a general macroscopic equation for the partial pressure in real gas mixtures, the product xiP is used as operational partial pressure. Using the virial expansion applied to the equation of state of nonideal gas mixtures truncated to the second order, we obtain an approximate equation of the partial pressures for such mixtures. This equation allows for a comparison with xiP as well as for the creation of diagrams expressing the total and partial pressures of the various constituents as a function of composition. Such diagrams prove useful to tackle this delicate subject with students, from a conceptual and from an educational point of view.
中文翻译:

计算真实气体混合物中分压的近似方程式
从物理角度来看,气体混合物中组分i的分压定义为分子i对混合物分子施加在壁上的每单位面积力的贡献。的关系p我= X我P(其中,X我是的摩尔分数我和P总压)在此定义为完美的气体的协议,但不是真正的气体。由于缺乏关于实际气体混合物中分压的一般宏观方程,因此将乘积x i P用作工作分压。使用应用于被截断为二阶的非理想气体混合物的状态方程的病毒膨胀,可以得到这种混合物的分压的近似方程。该方程式可以与x i P进行比较,还可以创建图表,将各种成分的总压力和分压表示为成分的函数。从概念和教育的角度来看,这些图表对于与学生一起解决这一微妙的问题都非常有用。
更新日期:2018-09-06
中文翻译:

计算真实气体混合物中分压的近似方程式
从物理角度来看,气体混合物中组分i的分压定义为分子i对混合物分子施加在壁上的每单位面积力的贡献。的关系p我= X我P(其中,X我是的摩尔分数我和P总压)在此定义为完美的气体的协议,但不是真正的气体。由于缺乏关于实际气体混合物中分压的一般宏观方程,因此将乘积x i P用作工作分压。使用应用于被截断为二阶的非理想气体混合物的状态方程的病毒膨胀,可以得到这种混合物的分压的近似方程。该方程式可以与x i P进行比较,还可以创建图表,将各种成分的总压力和分压表示为成分的函数。从概念和教育的角度来看,这些图表对于与学生一起解决这一微妙的问题都非常有用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号