当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diarylureas Containing 5-Membered Heterocycles as CB1 Receptor Allosteric Modulators: Design, Synthesis, and Pharmacological Evaluation.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-09-20 , DOI: 10.1021/acschemneuro.8b00396 Thuy Nguyen 1 , Thomas F Gamage 1 , Ann M Decker 1 , Nadezhda German 1 , Tiffany L Langston 1 , Charlotte E Farquhar 1 , Terry P Kenakin 2 , Jenny L Wiley 1 , Brian F Thomas 1 , Yanan Zhang 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-09-20 , DOI: 10.1021/acschemneuro.8b00396 Thuy Nguyen 1 , Thomas F Gamage 1 , Ann M Decker 1 , Nadezhda German 1 , Tiffany L Langston 1 , Charlotte E Farquhar 1 , Terry P Kenakin 2 , Jenny L Wiley 1 , Brian F Thomas 1 , Yanan Zhang 1
Affiliation
|
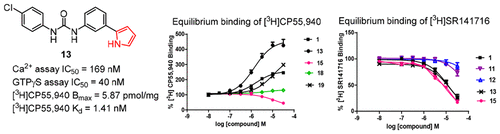
Allosteric modulators have attracted significant interest as an alternate strategy to modulate CB1 receptor signaling for therapeutic benefits that may avoid the adverse effects associated with orthosteric ligands. Here we extended our previous structure-activity relationship studies on the diarylurea-based CB1 negative allosteric modulators (NAMs) by introducing five-membered heterocycles to replace the 5-pyrrolidinylpyridinyl group in PSNCBAM-1 (1), one of the first generation CB1 allosteric modulators. Many of these compounds had comparable potency to 1 in blocking the CB1 agonist CP55,940 stimulated calcium mobilization and [35S]GTP-γ-S binding. Similar to 1, most compounds showed positive cooperativity by increasing [3H]CP55,940 binding, consistent with the positive allosteric modulator (PAM)-antagonist mechanism. Interestingly, these compounds exhibited differences in ability to increase specific binding of [3H]CP55,940 and decrease binding of the antagonist [3H]SR141716. In saturation binding studies, only increases in [3H]CP55,940 Bmax, but not Kd, were observed, suggesting that these compounds stabilize low affinity receptors into a high affinity state. Among the series, the 2-pyrrolyl analogue (13) exhibited greater potency than 1 in the [35S]GTP-γ-S binding assay and significantly enhanced the maximum binding level in the [3H]CP5,5940 binding assay, indicating greater CB1 receptor affinity and/or cooperativity.
中文翻译:

含有5元杂环作为CB1受体变构调节剂的二芳基脲:设计,合成和药理学评估。
变构调节剂作为调节CB1受体信号转导的替代策略引起了人们的极大兴趣,这种替代策略可避免与正构配体相关的副作用。在这里,我们通过引入五元杂环取代PSNCBAM-1(1)(第一代CB1变构之一)中的5-吡咯烷基吡啶基,扩展了以前基于二芳基脲的CB1负变构调节剂(NAM)的结构-活性关系研究。调制器。这些化合物中的许多在阻断CB1激动剂CP55,940刺激的钙动员和[35S]GTP-γ-S结合方面具有与1相当的效能。与1相似,大多数化合物通过增加[3H] CP55,940结合而显示出正的协同作用,这与正构变构调节剂(PAM)-拮抗剂机制相符。有趣的是,这些化合物在增加[3H] CP55,940的特异性结合和降低拮抗剂[3H] SR141716的结合方面表现出差异。在饱和结合研究中,仅观察到[3H] CP55,940 Bmax的增加,而未观察到Kd的增加,这表明这些化合物将低亲和力受体稳定为高亲和力状态。在该系列中,2-吡咯基类似物(13)在[35S]GTP-γ-S结合测定中显示出比1大的效力,并显着增强了[3H] CP5,5940结合测定中的最大结合水平,表明CB1更大受体亲和力和/或合作性。提示这些化合物将低亲和力受体稳定成高亲和力状态。在该系列中,2-吡咯基类似物(13)在[35S]GTP-γ-S结合测定中显示出比1大的效力,并显着增强了[3H] CP5,5940结合测定中的最大结合水平,表明CB1更大受体亲和力和/或合作性。提示这些化合物将低亲和力受体稳定成高亲和力状态。在该系列中,2-吡咯基类似物(13)在[35S]GTP-γ-S结合测定中显示出比1大的效力,并显着增强了[3H] CP5,5940结合测定中的最大结合水平,表明CB1更大受体亲和力和/或合作性。
更新日期:2018-09-06
中文翻译:

含有5元杂环作为CB1受体变构调节剂的二芳基脲:设计,合成和药理学评估。
变构调节剂作为调节CB1受体信号转导的替代策略引起了人们的极大兴趣,这种替代策略可避免与正构配体相关的副作用。在这里,我们通过引入五元杂环取代PSNCBAM-1(1)(第一代CB1变构之一)中的5-吡咯烷基吡啶基,扩展了以前基于二芳基脲的CB1负变构调节剂(NAM)的结构-活性关系研究。调制器。这些化合物中的许多在阻断CB1激动剂CP55,940刺激的钙动员和[35S]GTP-γ-S结合方面具有与1相当的效能。与1相似,大多数化合物通过增加[3H] CP55,940结合而显示出正的协同作用,这与正构变构调节剂(PAM)-拮抗剂机制相符。有趣的是,这些化合物在增加[3H] CP55,940的特异性结合和降低拮抗剂[3H] SR141716的结合方面表现出差异。在饱和结合研究中,仅观察到[3H] CP55,940 Bmax的增加,而未观察到Kd的增加,这表明这些化合物将低亲和力受体稳定为高亲和力状态。在该系列中,2-吡咯基类似物(13)在[35S]GTP-γ-S结合测定中显示出比1大的效力,并显着增强了[3H] CP5,5940结合测定中的最大结合水平,表明CB1更大受体亲和力和/或合作性。提示这些化合物将低亲和力受体稳定成高亲和力状态。在该系列中,2-吡咯基类似物(13)在[35S]GTP-γ-S结合测定中显示出比1大的效力,并显着增强了[3H] CP5,5940结合测定中的最大结合水平,表明CB1更大受体亲和力和/或合作性。提示这些化合物将低亲和力受体稳定成高亲和力状态。在该系列中,2-吡咯基类似物(13)在[35S]GTP-γ-S结合测定中显示出比1大的效力,并显着增强了[3H] CP5,5940结合测定中的最大结合水平,表明CB1更大受体亲和力和/或合作性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号