当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual catalysis system for ring-opening polymerization of lactones and 2,2-dimethyltrimethylene carbonate†
Polymer Chemistry ( IF 4.1 ) Pub Date : 2018-09-07 00:00:00 , DOI: 10.1039/c8py01230j Junhua Bai 1, 2, 3, 4 , Jinhua Wang 1, 2, 3, 4 , Yan Wang 1, 2, 3, 4 , Lifang Zhang 1, 2, 3, 4, 5
Polymer Chemistry ( IF 4.1 ) Pub Date : 2018-09-07 00:00:00 , DOI: 10.1039/c8py01230j Junhua Bai 1, 2, 3, 4 , Jinhua Wang 1, 2, 3, 4 , Yan Wang 1, 2, 3, 4 , Lifang Zhang 1, 2, 3, 4, 5
Affiliation
|
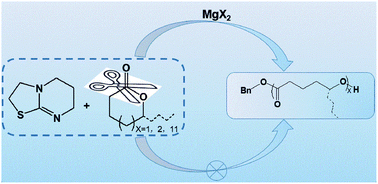
In this study, three isothioureas (ITUs), 2,3,5,6-tetrahydroimidazo[2,1-b]thiazole (ITU 1), 2,3,6,7-tetrahydro-5H-thiazolo-[3,2-a]pyrimidine (ITU 2) and 3,4,7,8-tetrahydro-2H,6H-pyrimido[2,1-b][1,3]thiazine (ITU 3) were prepared. These ITUs coupled with magnesium halides (MgX2) as the cocatalysts, cooperatively promoted the ring-opening polymerization (ROP) of several common lactones (including δ-valerolactone, ε-caprolactone, ε-decalactone and even of macrolactone ω-pentadecalactone) and 2,2-dimethyltrimethylene carbonate. These cocatalysts were found to exhibit suitable activities and ITU 2 emerged as the most active organic component. The order of activities for the magnesium halides was found to be lying in the following order: MgI2 > MgBr2 > MgCl2. The polymerizations attained high conversions (>90%) under optimal conditions, and produced linear polyesters having predictable molecular weights, narrow polydispersity indices (PDIs < 1.20) and defined end groups, which were derived from the benzyl alcohol initiator. The resulting polymers were characterized using GPC, IR, DSC, NMR and MALDI-ToF mass. The kinetic and chain extension experiments showed that the ITUs/MgX2-cocatalyzed ROPs of ε-caprolactone proceeded a living polymerization characteristics. Furthermore, the proposed polymerization mechanism was supposed to be a dual catalytic mechanism, and involved the activation of monomers through coordination to Lewis acids, while the initiator was activated by bases.
中文翻译:

内酯和2,2-二甲基三亚甲基碳酸酯开环聚合的双重催化系统†
在这项研究中,三个异硫脲(ITU),2,3,5,6-四氢咪唑并[2,1- b ]噻唑(ITU 1),2,3,6,7-四氢-5 H-噻唑并[3, 2-一个]嘧啶(ITU 2)和-3,4,7,8-四氢-2- ħ,6 ħ嘧啶并[2,1- b ] [1,3]噻嗪(ITU 3)中制备。这些ITU与卤化镁(MgX 2)作为助催化剂,共同促进了几种常见内酯(包括δ-戊内酯,ε-己内酯,ε-癸内酯甚至大内酯ω-十五内酯)和2,2-二甲基三亚甲基碳酸酯的开环聚合(ROP)。发现这些助催化剂表现出合适的活性,ITU 2成为最活跃的有机成分。发现卤化镁的活性顺序为以下顺序:MgI 2 > MgBr 2 > MgCl 2。在最佳条件下,聚合反应实现了高转化率(> 90%),并生产了具有可预测的分子量,窄的多分散指数(PDIs <1.20)和确定的端基的线性聚酯,这些线性聚酯衍生自苄醇引发剂。使用GPC,IR,DSC,NMR和MALDI-ToF质谱对所得聚合物进行表征。动力学和链增长实验表明,ITUs / MgX 2催化的ε-己内酯的ROP进行了活性聚合反应。此外,所提出的聚合机理被认为是双重催化机理,并且涉及通过与路易斯酸的配位来活化单体,而引发剂则通过碱来活化。
更新日期:2018-09-07
中文翻译:

内酯和2,2-二甲基三亚甲基碳酸酯开环聚合的双重催化系统†
在这项研究中,三个异硫脲(ITU),2,3,5,6-四氢咪唑并[2,1- b ]噻唑(ITU 1),2,3,6,7-四氢-5 H-噻唑并[3, 2-一个]嘧啶(ITU 2)和-3,4,7,8-四氢-2- ħ,6 ħ嘧啶并[2,1- b ] [1,3]噻嗪(ITU 3)中制备。这些ITU与卤化镁(MgX 2)作为助催化剂,共同促进了几种常见内酯(包括δ-戊内酯,ε-己内酯,ε-癸内酯甚至大内酯ω-十五内酯)和2,2-二甲基三亚甲基碳酸酯的开环聚合(ROP)。发现这些助催化剂表现出合适的活性,ITU 2成为最活跃的有机成分。发现卤化镁的活性顺序为以下顺序:MgI 2 > MgBr 2 > MgCl 2。在最佳条件下,聚合反应实现了高转化率(> 90%),并生产了具有可预测的分子量,窄的多分散指数(PDIs <1.20)和确定的端基的线性聚酯,这些线性聚酯衍生自苄醇引发剂。使用GPC,IR,DSC,NMR和MALDI-ToF质谱对所得聚合物进行表征。动力学和链增长实验表明,ITUs / MgX 2催化的ε-己内酯的ROP进行了活性聚合反应。此外,所提出的聚合机理被认为是双重催化机理,并且涉及通过与路易斯酸的配位来活化单体,而引发剂则通过碱来活化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号