当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, characterization, and evaluation of iron nanoparticles as hydrogenation catalysts in alcohols and tetraalkylphosphonium ionic liquids: do solvents matter?
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-09-06 00:00:00 , DOI: 10.1039/c8cy01346b Abhinandan Banerjee 1, 2, 3, 4 , Yali Yao 1, 2, 3, 4 , Michael-Roy R. Durr 1, 2, 3, 4 , William G. Barrett 1, 2, 3, 4 , Yongfeng Hu 2, 3, 4, 5 , Robert W. J. Scott 1, 2, 3, 4
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-09-06 00:00:00 , DOI: 10.1039/c8cy01346b Abhinandan Banerjee 1, 2, 3, 4 , Yali Yao 1, 2, 3, 4 , Michael-Roy R. Durr 1, 2, 3, 4 , William G. Barrett 1, 2, 3, 4 , Yongfeng Hu 2, 3, 4, 5 , Robert W. J. Scott 1, 2, 3, 4
Affiliation
|
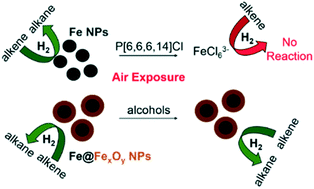
Iron nanoparticles (Fe NPs) synthesized via a one-pot chemical reduction strategy in neat alcohol or water/alcohol mixtures are compared to and contrasted with those synthesized in tetraalkylphosphonium ionic liquids with respect to their catalytic activities for the hydrogenation of simple olefins. It was observed that Fe NPs could catalyze the conversion of 2-norbornene to norbornane and 1-octene to octane in good yields under moderately high hydrogen pressures. Core–shell type Fe@FexOy particles in alcoholic solvents with larger sizes show greater resistance to catalytic deactivation after several reaction cycles, whereas halide ionic liquid-capped Fe particles show a marked tendency towards oxidative degradation, which limits their utility in catalysis unless rigorous anhydrous and anoxic conditions are maintained. Finally, in situ X-ray absorption spectroscopy was applied to determine the fate of these systems upon catalysis and exposure to air. No change in Fe speciation was seen for Fe@FexOy nanoparticles in alcohol solvents. Fe nanoparticles in ionic liquids with strongly coordinating anions such as halides were not particularly stable to oxidation, while those in ionic liquids with non-coordinating anions agglomerate during catalysis, as well as undergoing slow oxidative degradation, thus making these systems less useful for catalysis compared to their counterparts in alcoholic solvents.
中文翻译:

醇和四烷基phosph离子液体中作为氢化催化剂的铁纳米粒子的合成,表征和评估:溶剂重要吗?
将通过一锅化学还原策略在纯醇或水/醇混合物中合成的铁纳米颗粒(Fe NPs)与在四烷基phosph离子液体中合成的铁纳米颗粒的催化活性进行了比较,并与之进行了对比。观察到,在中等高的氢气压力下,Fe NPs可以良好的产率催化2-降冰片烯向降冰片烷的转化和1-辛烯向辛烷的转化。核-壳型Fe @ Fe x O y在几个反应循环后,较大尺寸的醇类溶剂中的颗粒显示出更大的抗催化失活性,而卤化物离子液体封端的Fe颗粒则表现出明显的氧化降解趋势,除非维持严格的无水和缺氧条件,否则它们在催化中的应用受到限制。最后,采用原位X射线吸收光谱法确定这些系统在催化和暴露于空气后的命运。Fe @ Fe x O y的铁形态未见变化醇溶剂中的纳米颗粒。具有强配位阴离子(例如卤化物)的离子液体中的铁纳米颗粒对氧化不是特别稳定,而具有非配位阴离子的离子液体中的Fe纳米颗粒在催化过程中会聚结,并且会经历缓慢的氧化降解,因此与这些体系相比,这些系统对催化的作用较小到他们在酒精溶剂中的对应物。
更新日期:2018-09-06
中文翻译:

醇和四烷基phosph离子液体中作为氢化催化剂的铁纳米粒子的合成,表征和评估:溶剂重要吗?
将通过一锅化学还原策略在纯醇或水/醇混合物中合成的铁纳米颗粒(Fe NPs)与在四烷基phosph离子液体中合成的铁纳米颗粒的催化活性进行了比较,并与之进行了对比。观察到,在中等高的氢气压力下,Fe NPs可以良好的产率催化2-降冰片烯向降冰片烷的转化和1-辛烯向辛烷的转化。核-壳型Fe @ Fe x O y在几个反应循环后,较大尺寸的醇类溶剂中的颗粒显示出更大的抗催化失活性,而卤化物离子液体封端的Fe颗粒则表现出明显的氧化降解趋势,除非维持严格的无水和缺氧条件,否则它们在催化中的应用受到限制。最后,采用原位X射线吸收光谱法确定这些系统在催化和暴露于空气后的命运。Fe @ Fe x O y的铁形态未见变化醇溶剂中的纳米颗粒。具有强配位阴离子(例如卤化物)的离子液体中的铁纳米颗粒对氧化不是特别稳定,而具有非配位阴离子的离子液体中的Fe纳米颗粒在催化过程中会聚结,并且会经历缓慢的氧化降解,因此与这些体系相比,这些系统对催化的作用较小到他们在酒精溶剂中的对应物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号