Journal of Power Sources ( IF 8.1 ) Pub Date : 2018-09-05 , DOI: 10.1016/j.jpowsour.2018.08.086 Ruirun Chen , Xin Ding , Xiaoyu Chen , Xinzhong Li , Jingjie Guo , Yanqing Su , Hongsheng Ding , Hengzhi Fu
|
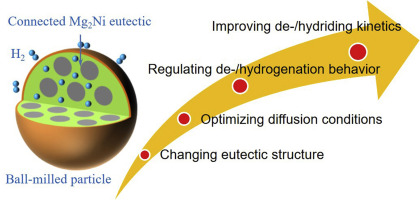
To illuminate the effects of eutectic structure on the de-/hydrogenation properties of Mg-based alloy, Mg-9Ni, Mg-12Cu and Mg-6Ni-3Cu (at. %) alloys with nearly the same primary Mg proportion are prepared. Mg2Ni and Mg2Cu respectively form a eutectic structure with Mg and tunable eutectic is obtained via Cu partial substituting Ni. Mg-6Ni-3Cu alloy shows excellent activation performance and rapid absorption rates without any sacrifice of desorption rates, leading to a maximum hydrogen capacity of 6.5 wt% H2 under 300 °C 3 MPa. Different from the independent de-/hydriding behavior of Mg->12Cu alloy, Mg-6Ni-3Cu alloy shows perfectly wide pressure-composition-isotherm plateau with minimal slope. The dehydrogenation models of experimental hydrides are determined. Owing to Cu substitution, the dehydrogenation activation energy reduces from 95.82 to 67.34 kJ mol−1 H2, the onset/peak desorption temperature also reduces significantly. It is the spatial connected eutectic and its increased boundaries that facilitate the activation and H diffusion process, which greatly compensate the negative impacts of reduced hydrogenation driving force caused by Mg2Cu. The rapid H diffusion coupled with the increased dehydrogenation driving force collectively facilitates its dehydriding process.
中文翻译:

通过Mg-Ni合金中的Cu替代实现可调节的共晶结构和脱氢/氢化行为
为了阐明共晶结构对Mg基合金的脱氢/加氢性能的影响,制备了具有几乎相同的主Mg比例的Mg-9Ni,Mg-12Cu和Mg-6Ni-3Cu(原子%)合金。Mg 2 Ni和Mg 2 Cu分别与Mg形成共晶结构,并且通过Cu部分替代Ni获得可调谐的共晶。Mg-6Ni-3Cu合金具有出色的活化性能和快速吸收速率,而不会牺牲任何解吸速率,从而导致最大氢容量为6.5 wt%H 2在300°C下3 MPa。与Mg-> 12Cu合金的独立脱/氢化行为不同,Mg-6Ni-3Cu合金表现出非常宽的压力组成-等温线平稳区,斜率最小。确定了实验氢化物的脱氢模型。由于Cu的取代,脱氢活化能从95.82降低至67.34kJ mol -1 H 2,起始/峰解吸温度也显着降低。是空间连接的共晶及其增加的边界,促进了活化和氢扩散过程,极大地补偿了由Mg 2引起的加氢驱动力降低带来的负面影响。铜 快速的H扩散和增加的脱氢驱动力共同促进了其脱水过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号