当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Studies on Biosynthetic Carbocation Rearrangements Leading to Quiannulatene: Initial Conformation Regulates Biosynthetic Route, Stereochemistry, and Skeleton Type
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-10-11 , DOI: 10.1002/anie.201807139 Hajime Sato 1, 2 , Takaaki Mitsuhashi 3 , Mami Yamazaki 1 , Ikuro Abe 3 , Masanobu Uchiyama 2, 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-10-11 , DOI: 10.1002/anie.201807139 Hajime Sato 1, 2 , Takaaki Mitsuhashi 3 , Mami Yamazaki 1 , Ikuro Abe 3 , Masanobu Uchiyama 2, 3
Affiliation
|
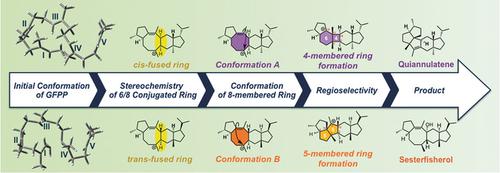
The results of quantum chemical calculations on the mechanism of the carbocation cascade of reactions in the biosynthetic pathways leading to the pentacyclic sesterterpenes quiannulatene and sesterfisherol provide reasonable answers to several persistent mechanistic questions in sesterterpene biosynthesis, including: 1) the reaction pathways of the multicyclic ring system construction and skeletal rearrangements, 2) the mechanism of triquinane skeleton formation, which requires more complicated rearrangements than previously proposed, 3) the stereochemistry of the final carbocation intermediate, and 4) the determining factor of biosynthetic selection for either 5/6/4/6/5 or 5/6/5/5/5 pentacyclic skeleton formation. This in‐depth mechanistic study on sesterterpene biosynthesis revealed that the shape of the final product and the type of triquinane skeleton formed are regulated by the stereochemistry and conformation of the common starting material, geranylfarnesyl diphosphate (GFPP).
中文翻译:

导致喹咯烷的生物合成碳正离子重排的计算研究:初始构象调节生物合成途径,立体化学和骨架类型。
碳化学反应在碳合成级联反应机理上的量子化学计算结果,导致五环酯基酯和戊烯醇的合成提供了对酯基酯生物合成中若干持久性机理问题的合理答案,其中包括:1)多环环的反应途径系统构造和骨架重排,2)三喹烷骨架形成的机理,与先前提出的相比,它需要更复杂的重排,3)最终碳正离子中间体的立体化学,4)5/6/4的生物合成选择的决定因素/ 6/5或5/6/5/5/5五环骨架形成。这项关于酯化萜烯生物合成的深入机理研究表明,最终产物的形状和所形成的三喹烷骨架的类型受常见起始原料香叶基法呢基二磷酸酯(GFPP)的立体化学和构象调节。
更新日期:2018-10-11
中文翻译:

导致喹咯烷的生物合成碳正离子重排的计算研究:初始构象调节生物合成途径,立体化学和骨架类型。
碳化学反应在碳合成级联反应机理上的量子化学计算结果,导致五环酯基酯和戊烯醇的合成提供了对酯基酯生物合成中若干持久性机理问题的合理答案,其中包括:1)多环环的反应途径系统构造和骨架重排,2)三喹烷骨架形成的机理,与先前提出的相比,它需要更复杂的重排,3)最终碳正离子中间体的立体化学,4)5/6/4的生物合成选择的决定因素/ 6/5或5/6/5/5/5五环骨架形成。这项关于酯化萜烯生物合成的深入机理研究表明,最终产物的形状和所形成的三喹烷骨架的类型受常见起始原料香叶基法呢基二磷酸酯(GFPP)的立体化学和构象调节。









































 京公网安备 11010802027423号
京公网安备 11010802027423号