当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational synthesis of benzimidazole[3]arenes by CuII-catalyzed post-macrocyclization transformation†‡
Chemical Science ( IF 7.6 ) Pub Date : 2018-09-05 00:00:00 , DOI: 10.1039/c8sc03086c Shohei Tashiro 1, 2, 3, 4, 5 , Tsutomu Umeki 1, 2, 3, 4, 5 , Ryou Kubota 1, 2, 3, 4, 5 , Mitsuhiko Shionoya 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2018-09-05 00:00:00 , DOI: 10.1039/c8sc03086c Shohei Tashiro 1, 2, 3, 4, 5 , Tsutomu Umeki 1, 2, 3, 4, 5 , Ryou Kubota 1, 2, 3, 4, 5 , Mitsuhiko Shionoya 1, 2, 3, 4, 5
Affiliation
|
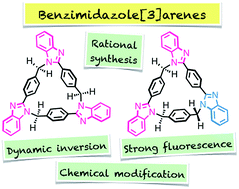
A new series of calix[n]arene analogues, benzimidazole[3]arenes, was rationally synthesized by CuII-catalyzed post-macrocyclization transformation of a tris(o-phenylenediamine) macrocycle, and fully characterized by NMR, MS, and single-crystal X-ray diffraction (XRD) analyses. The resulting syn- and anti-benzimidazole[3]arenes have a bowl-shaped and a warped structure, respectively, in their crystalline states, and both display a dynamic inversion behavior in solution. This modification resulted in strong fluorescence due to the generated benzimidazole moieties. The mechanistic study of the post-macrocyclization transformation demonstrated that the formation of both benzimidazole[3]arenes was catalyzed, via triimine intermediates, by CuII ions in air through oxidation and cyclization of the tris(o-phenylenediamine) macrocycle.
中文翻译:

苯并咪唑的合成理性[3]用Cu芳烃II催化的大环化后变换†往最‡
通过Cu II催化的三(邻苯二胺)大环的大环环化反应合理合成了一系列新的杯芳烃[ n ]芳烃类似物苯并咪唑[3]芳烃,并通过NMR,MS和单-晶体X射线衍射(XRD)分析。所得的顺式和反式苯并咪唑[3]芳烃在其结晶态下分别具有碗形和翘曲结构,并且在溶液中均显示出动态的反转行为。由于产生的苯并咪唑部分,这种修饰导致强烈的荧光。宏环化后转化的机理研究表明,苯并咪唑[3]芳烃的形成均被催化,通过三亚胺中间体,通过空气中的Cu II离子通过三(邻苯二胺)大环的氧化和环化作用。
更新日期:2018-09-05
中文翻译:

苯并咪唑的合成理性[3]用Cu芳烃II催化的大环化后变换†往最‡
通过Cu II催化的三(邻苯二胺)大环的大环环化反应合理合成了一系列新的杯芳烃[ n ]芳烃类似物苯并咪唑[3]芳烃,并通过NMR,MS和单-晶体X射线衍射(XRD)分析。所得的顺式和反式苯并咪唑[3]芳烃在其结晶态下分别具有碗形和翘曲结构,并且在溶液中均显示出动态的反转行为。由于产生的苯并咪唑部分,这种修饰导致强烈的荧光。宏环化后转化的机理研究表明,苯并咪唑[3]芳烃的形成均被催化,通过三亚胺中间体,通过空气中的Cu II离子通过三(邻苯二胺)大环的氧化和环化作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号