当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aliphatic C—H Oxidations for Late-Stage Functionalization
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-09-05 , DOI: 10.1021/jacs.8b05195 M Christina White 1 , Jinpeng Zhao 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-09-05 , DOI: 10.1021/jacs.8b05195 M Christina White 1 , Jinpeng Zhao 1
Affiliation
|
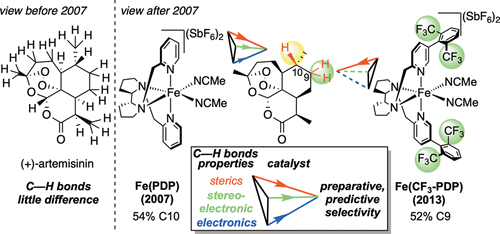
The atomistic change of C( sp3)-H to C( sp3)-O can have a profound impact on the physical and biological properties of small molecules. Traditionally, chemical synthesis has relied on pre-existing functionality to install new functionality, and directed approaches to C-H oxidation are an extension of this logic. The impact of developing undirected C-H oxidation reactions with controlled site-selectivity is that scientists gain the ability to diversify complex structures at sites remote from existing functionality, without having to carry out individual de novo syntheses. This Perspective offers a historical view of why, as recently as 2007, it was thought that the differences between aliphatic C-H bonds of the same bond type (for example, 2° aliphatic) were not large enough to distinguish them preparatively with small-molecule catalysis in the absence of directing groups or molecular recognition elements. We give an account of the discovery of Fe(PDP)-catalyzed non-directed aliphatic C-H hydroxylations and how the electronic, steric, and stereoelectronic rules for predicting site-selectivity that emerged have affected a shift in how the chemical community views the reactivity among these bonds. The discovery that site-selectivity could be altered by tuning the catalyst [i.e., Fe(CF3-PDP)] with no changes to the substrate or reaction now gives scientists the ability to exert control on the site of oxidation on a range of functionally and topologically diverse compounds. Collectively, these findings have made possible the emerging area of late-stage C-H functionalizations for streamlining synthesis and derivatizing complex molecules.
中文翻译:

用于后期功能化的脂肪族 C—H 氧化
C(sp3)-H到C(sp3)-O的原子变化可以对小分子的物理和生物性质产生深远的影响。传统上,化学合成依赖于预先存在的功能来安装新功能,而 CH 氧化的定向方法是这种逻辑的延伸。开发具有受控位点选择性的无向CH氧化反应的影响是,科学家们能够在远离现有功能的位点实现复杂结构的多样化,而无需进行单独的从头合成。本视角提供了历史观点,说明为什么直到 2007 年,人们还认为相同键类型(例如 2° 脂肪族)的脂肪族 CH 键之间的差异不足以通过小分子催化来区分它们在没有导向基团或分子识别元件的情况下。我们描述了 Fe(PDP) 催化的非定向脂肪族 CH 羟基化的发现,以及预测位点选择性的电子、空间和立体电子规则如何影响化学界如何看待化学反应性的转变这些债券。通过调整催化剂 [即 Fe(CF3-PDP)] 可以在不改变底物或反应的情况下改变位点选择性的发现,现在使科学家能够在一系列功能和功能上对氧化位点进行控制。拓扑多样化的化合物。总的来说,这些发现使得后期 CH 官能化的新兴领域成为可能,以简化合成和衍生复杂分子。
更新日期:2018-09-05
中文翻译:

用于后期功能化的脂肪族 C—H 氧化
C(sp3)-H到C(sp3)-O的原子变化可以对小分子的物理和生物性质产生深远的影响。传统上,化学合成依赖于预先存在的功能来安装新功能,而 CH 氧化的定向方法是这种逻辑的延伸。开发具有受控位点选择性的无向CH氧化反应的影响是,科学家们能够在远离现有功能的位点实现复杂结构的多样化,而无需进行单独的从头合成。本视角提供了历史观点,说明为什么直到 2007 年,人们还认为相同键类型(例如 2° 脂肪族)的脂肪族 CH 键之间的差异不足以通过小分子催化来区分它们在没有导向基团或分子识别元件的情况下。我们描述了 Fe(PDP) 催化的非定向脂肪族 CH 羟基化的发现,以及预测位点选择性的电子、空间和立体电子规则如何影响化学界如何看待化学反应性的转变这些债券。通过调整催化剂 [即 Fe(CF3-PDP)] 可以在不改变底物或反应的情况下改变位点选择性的发现,现在使科学家能够在一系列功能和功能上对氧化位点进行控制。拓扑多样化的化合物。总的来说,这些发现使得后期 CH 官能化的新兴领域成为可能,以简化合成和衍生复杂分子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号